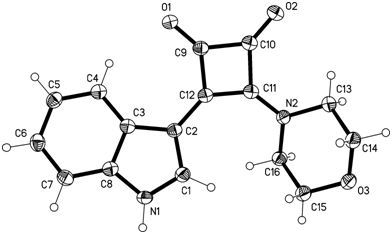
3-(1H-Indol-3-yl)-4-(morpholin-4-yl)cyclobut-3-ene-1,2-dione
Abstract
:Experimental
General
3-(1H-Indol-3-yl)-4-(morpholin-4-yl)cyclobut-3-ene-1,2-dione (3)
X-Ray Structure Determination of 3
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Marson, C.M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 2011, 40, 5514–5533. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bunnelle, W.H.; Ryther, K.B.; Anderson, D.J.; Malysz, J.; Helfrich, R.; Grønlien, J.H.; Håkerud, M.; Peters, D.; Schrimpf, M.R.; et al. Syntheses and structure-activity relationship (SAR) studies of 2,5-diazabicyclo[2.2.1]heptanes as novel alpha7 neuronal nicotinic receptor (NNR) ligands. Bioorg. Med. Chem. Lett. 2010, 20, 3636–3639. [Google Scholar] [CrossRef] [PubMed]
- Kunick, C.; Zeng, Z.; Gussio, R.; Zaharevitz, D.; Leost, M.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Meijer, L.; Lemcke, T. Structure-aided optimization of kinase inhibitors derived from alsterpaullone. ChemBioChem 2005, 6, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Reichwald, C.; Shimony, O.; Dunkel, U.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Kunick, C. 2-(3-Aryl-3-oxopropen-1-yl)-9-tert-butyl-paullones: A new antileishmanial chemotype. J. Med. Chem. 2008, 51, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Wieking, K.; Knockaert, M.; Leost, M.; Zaharevitz, D.W.; Meijer, L.; Kunick, C. Synthesis of paullones with aminoalkyl side chains. Arch. Pharm. Pharm. Med. Chem. 2002, 335, 311–317. [Google Scholar] [CrossRef]
- Xie, X.; Lemcke, T.; Gussio, R.; Zaharevitz, D.W.; Leost, M.; Meijer, L.; Kunick, C. Epoxide-containing side chains enhance antiproliferative activity of paullones. Eur. J. Med. Chem. 2005, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Preu, L.; Lemcke, T.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Kunick, C. Dual IGF-1R/SRC inhibitors based on a N'-aroyl-2-(1H-indol-3-yl)-2-oxoacetohydrazide structure. Eur. J. Med. Chem. 2011, 46, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Storer, R.I.; Aciroa, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef] [PubMed]
- Shinada, T.; Nakagawa, Y.; Hayashi, K.; Corzo, G.; Nakajima, T.; Ohfune, Y. Synthesis and paralytic activities of squaryl amino acid-containing polyamine toxins. Amino Acids 2003, 24, 293–301. [Google Scholar] [PubMed]
- Charton, J.; Déprez, B.P.; Déprez-Poulain, R.F. Synthesis of a 200-member library of squaric acid N-hydroxylamide amides. Bioorg. Med. Chem. Lett. 2008, 18, 4968–4971. [Google Scholar] [CrossRef] [PubMed]
- Arrington, K.L.; Dudkin, V.Y. Novel inhibitors of checkpoint kinase 1. ChemMedChem 2007, 2, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H.; Thiel, S.H.; Gaschler, O. Oxocarbons and related compounds. Part 24. Chlorosquarylation of indoles. J. Chem. Soc. Perkin Trans. 1 1996, 495–496. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]


| Run | Molar Ratio indolylsquarylchloride (1):morpholine (2) | Moles triethylamine | Solvent | Yield [%] |
| 1 | 1:1 | 0 | ethanol | 46.2 |
| 2 | 1:2.2 | 0 | ethanol | 82.3 |
| 3 | 1:1 | 1 | ethanol | 70.5 |
| 4 | 1:1 | 0 | acetonitrile | 47.2 |
| 5 | 1:2.2 | 0 | acetonitrile | 73.2 |
| 6 | 1:1 | 1 | acetonitrile | 65.3 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maiwald, F.; Jones, P.G.; Aref, M.; Böttcher, M.; Karwehl, M.; Schniers, A.; Stanke, C.; Grünefeld, J. 3-(1H-Indol-3-yl)-4-(morpholin-4-yl)cyclobut-3-ene-1,2-dione. Molbank 2012, 2012, M758. https://doi.org/10.3390/M758
Maiwald F, Jones PG, Aref M, Böttcher M, Karwehl M, Schniers A, Stanke C, Grünefeld J. 3-(1H-Indol-3-yl)-4-(morpholin-4-yl)cyclobut-3-ene-1,2-dione. Molbank. 2012; 2012(2):M758. https://doi.org/10.3390/M758
Chicago/Turabian StyleMaiwald, Franziska, Peter G. Jones, Mahmoud Aref, Marina Böttcher, Marc Karwehl, Armin Schniers, Carmen Stanke, and Johann Grünefeld. 2012. "3-(1H-Indol-3-yl)-4-(morpholin-4-yl)cyclobut-3-ene-1,2-dione" Molbank 2012, no. 2: M758. https://doi.org/10.3390/M758