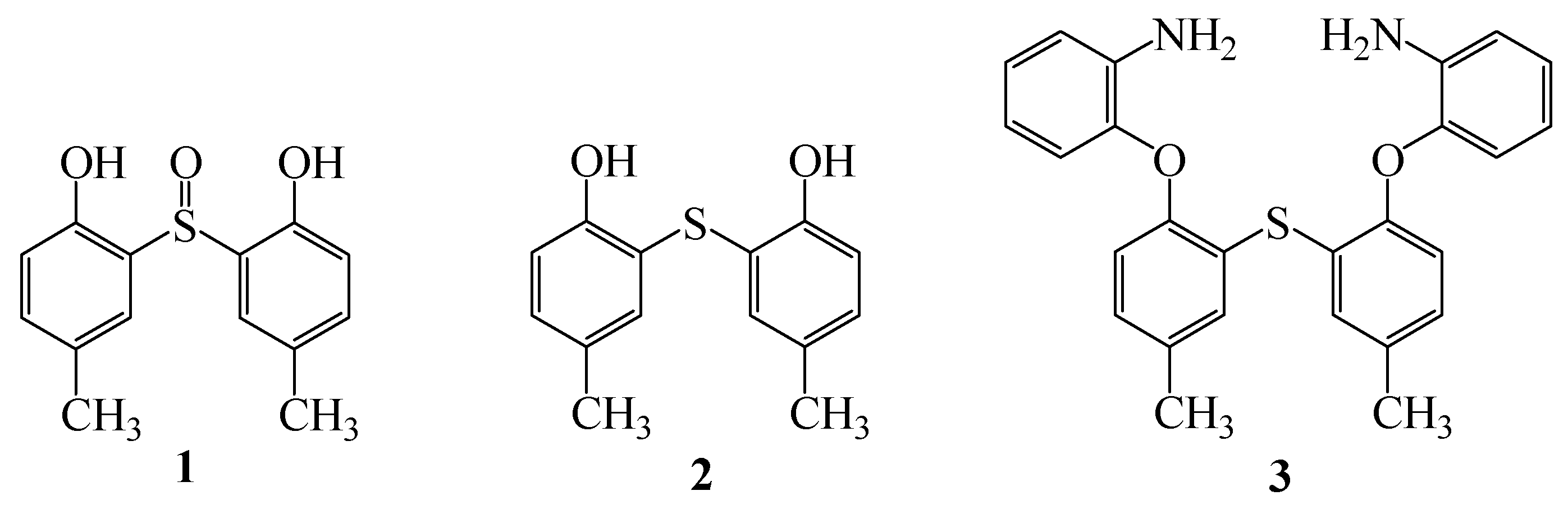
2,2'-Thio-bis[(4-methylphenyl)-2-aminobenzoate]
Abstract
:Introduction
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Gazdar, M.; Smiles, S. Aromatic hydroxy-sulphoxides. J. Chem. Soc. Trans. 1910, 97, 2248–2253. [Google Scholar] [CrossRef]
- Gump, W.S.; Vitucci, J.C. 2-Hydroxyphenyl sulfoxides and 2-hydroxyphenyl sulfones. J. Am. Chem. Soc. 1945, 67, 238–240. [Google Scholar] [CrossRef]
- Jung, M.E.; Jachiet, D.; Khan Saeed, I.; Kim, C. New method for the preparation of o-aryloxyphenols: Pummerer-type rearrangement of an o-hydroxyaryl sulfoxide. Tetrahedron Lett. 1995, 36, 361–364. [Google Scholar] [CrossRef]
- Cogan, D.A.; Liu, G.; Kim, K.; Backes, B.J.; Ellman, J.A. Catalytic asymmetric oxidation of tert-butyl disulfide. synthesis of tert-butanesulfinamides, tert-butyl sulfoxides, and tert-butanesulfinimines. J. Am. Chem. Soc. 1998, 120, 8011–8019. [Google Scholar] [CrossRef]
- Schmidt, H.; Bashirpoor, M.; Rehder, D. Structural characterization of possible intermediates in vanadium-catalysed sulfide oxidation. J. Chem. Soc. Dalton Trans. 1996, 3865–3870. [Google Scholar] [CrossRef]
- Jouen, C.; Lasne, M.C.; Pammelet, J.C. Synthesis of α-fluorinated-α,α-difunctionalized sulfides and sulfones. Tetrahedron Lett. 1996, 37, 2413–2416. [Google Scholar] [CrossRef]
- Cubbage, J.W.; Tetzlaff, T.A.; Groundwater, H.; McCulla, R.D.; Nog, M.; Jenks, W.S. Bimolecular photoreduction of aromatic sulfoxides. J. Org. Chem. 2001, 66, 8621–8628. [Google Scholar] [CrossRef] [PubMed]
- Shockravi, A.; Alizadeh, R.; Aghabozorg, H.; Moghimi, A.; Rostami, E.; Bavilli, S. Synthesis and crystal structure determination of 2,2'-sulfinyl-bis(4-methyl phenol) and 2,2'-thio-bis (4-methyl phenol). Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 2519–2527. [Google Scholar] [CrossRef]
- Shockravi, A.; Chaloosi, M.; Zakeri, M.; Mozaffari, S.; Rostami, E.; Abouzari- Lotf, E. The synthesis and characterization of novel dibenzosulfide diamine and the application in the determination of heavy metals. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2321–2326. [Google Scholar] [CrossRef]
- Shockravi, A.; Sadeghpour, M.; Olyaei, A. Solvent-and catalyst-free synthesis of new unsymmetrical multidentate thio-bis aminophenol ligands by Mannich condensation. Synth. Commun. 2009, 39, 2347–2359. [Google Scholar] [CrossRef]
- Shockravi, A.; Sadeghpour, M.; Zakeri, M.; Abouzari-Lotf, E.; Olyaei, A. Synthesis of new multibenzo oxygen-sulfur donor macrocycles containing lactams at room temperature. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 808–815. [Google Scholar] [CrossRef]

© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sadeghpour, M.; Olyaei, A.; Rezaei, M. 2,2'-Thio-bis[(4-methylphenyl)-2-aminobenzoate]. Molbank 2012, 2012, M760. https://doi.org/10.3390/M760
Sadeghpour M, Olyaei A, Rezaei M. 2,2'-Thio-bis[(4-methylphenyl)-2-aminobenzoate]. Molbank. 2012; 2012(2):M760. https://doi.org/10.3390/M760
Chicago/Turabian StyleSadeghpour, Mahdieh, Abolfazl Olyaei, and Mortaza Rezaei. 2012. "2,2'-Thio-bis[(4-methylphenyl)-2-aminobenzoate]" Molbank 2012, no. 2: M760. https://doi.org/10.3390/M760




