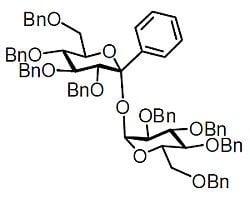
2,3,4,6-Tetra-O-benzyl-1-C-phenyl-α-D-glucopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside
Abstract
:Experimental
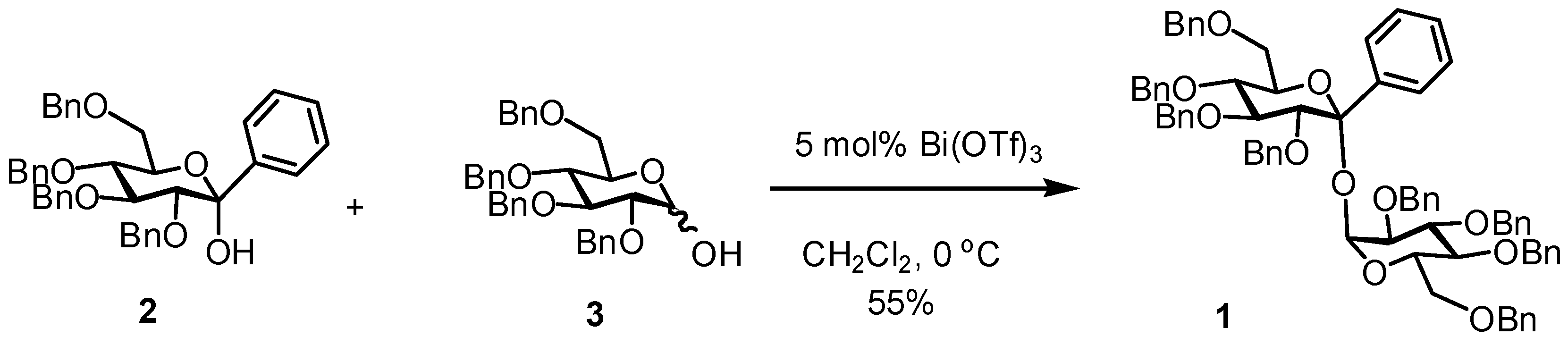
2,3,4,6-Tetra-O-benzyl-1-C-phenyl-α-d-glucopyranosyl 2,3,4,6-tetra-O-benzyl-α-d-glucopyranoside (1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Hiruma, K.; Kajimoto, T.; Weitz-Schmidt, G.; Ollmann, I.; Wong, C.-H. Rational design and synthesis of a 1,1-linked disaccharide that is 5 times as active as sialyl Lewis X in binding to E-selectin. J. Am. Chem. Soc. 1996, 118, 9265–9270. [Google Scholar] [CrossRef]
- Dohi, H.; Nishida, Y.; Furuta, Y.; Uzawa, H.; Yokoyama, S.-I.; Ito, S.; Mori, H.; Kobayashi, K. Molecular design and biological potential of galacto-type trehalose as a nonnatural ligand of shiga toxins. Org. Lett. 2002, 4, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chang, C.-H.T. Convenient divergent synthesis of a library of trehalosamine analogues. Org. Lett. 2002, 4, 2245–2248. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Inoue, R.; Matsuda, S.; Hamasaki, K. Bismuth(III) triflate-catalyzed dehydrative glycosidation using 1-hydroxy sugars. Lett. Org. Chem. 2008, 5, 30–33. [Google Scholar] [CrossRef]
- Yamanoi, T.; Inoue, R.; Matsuda, S.; Iwao, K.; Oda, Y.; Yoshida, A.; Hamasaki, K. Formation of O-glycosidic linkages from 1-hydroxy sugars by bismuth(III) triflate-catalyzed dehydrative glycosidation. Heterocycles 2009, 77, 445–460. [Google Scholar] [CrossRef]
- Yamanoi, T.; Inoue, R.; Matsuda, S.; Katsuraya, K.; Hamasaki, K. Synthesis of trehalose mimics by bismuth(III) triflate or bis(trifluoromethane)sulfonimide-catalyzed 1-C-methyl-d-hexopyranosylation. Tetrahedron Asymmetry 2006, 17, 2914–2918. [Google Scholar] [CrossRef]
- Yamanoi, T.; Inoue, R.; Oda, Y.; Hamasaki, K. 6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-d-gluco-dec-5-ulopyranosyl 2,3,4,6-tetra-O-benzyl-α-d-glucopyranoside. Molbank 2010, 2010, M671. [Google Scholar] [CrossRef]
- Ellsworth, B.-A.; Doyle, A.-G.; Patel, M.; Caceres-Cortes, J.; Meng, W.; Deshpande, P.-P.; Pullockaran, A.; Washburn, W.-N. C-Arylglucoside synthesis: Triisopropylsilane as a selective reagent for the reduction of an anomeric C-phenyl ketal. Tetrahedron Asymmetry 2003, 14, 3243–3247. [Google Scholar] [CrossRef]
- Czernecki, S.; Ville, G. C-Glycosides. 7. Stereospecific C-glycosylation of aromatic and heterocyclic rings. J. Org. Chem. 1989, 54, 610–612. [Google Scholar] [CrossRef]
- Yamanoi, T.; Misawa, N.; Matsuda, S.; Watanabe, M. Preparation of partially benzylated mono-, di-, and trisaccharides by selective cleavage of the β-fructofuranosidic linkage in fully benzylated sucrose and sucrose-related oligosaccharides under acidic conditions. Carbohydr. Res. 2008, 343, 1366–1372. [Google Scholar] [CrossRef] [PubMed]

© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamanoi, T.; Inoue, R.; Oda, Y. 2,3,4,6-Tetra-O-benzyl-1-C-phenyl-α-D-glucopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside. Molbank 2012, 2012, M761. https://doi.org/10.3390/M761
Yamanoi T, Inoue R, Oda Y. 2,3,4,6-Tetra-O-benzyl-1-C-phenyl-α-D-glucopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside. Molbank. 2012; 2012(2):M761. https://doi.org/10.3390/M761
Chicago/Turabian StyleYamanoi, Takashi, Ryo Inoue, and Yoshiki Oda. 2012. "2,3,4,6-Tetra-O-benzyl-1-C-phenyl-α-D-glucopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside" Molbank 2012, no. 2: M761. https://doi.org/10.3390/M761