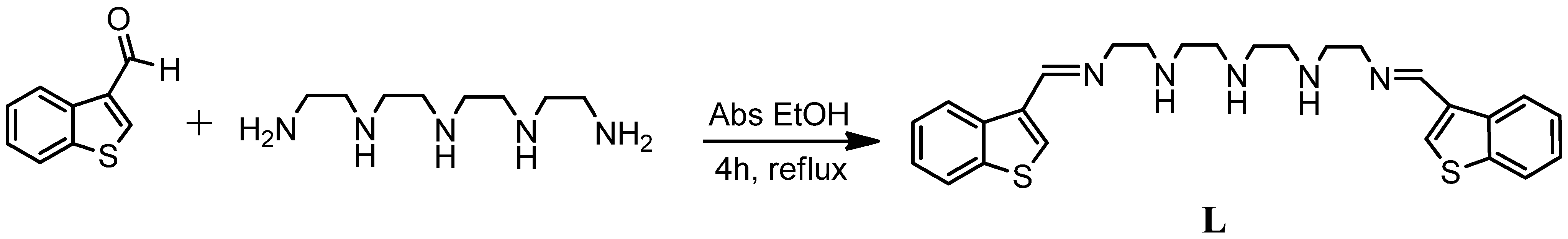
N1-(Benzo[b]thiophen-3-ylmethylidene)-N2-(2-((2-((2-(benzo[b]thiophen-3-ylmethylidene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Sall, D.J.; Bailey, D.L.; Bastian, J.A.; Buben, J.A.; Chirgadze, N.Y.; Clemens-Smith, A.C.; Denney, M.L.; Fisher, M.J.; Giera, D.D.; Gifford-Moore, D.S.; et al. Diamino Benzo[b]thiophene Derivatives as a Novel Class of Active Site Directed Thrombin Inhibitors. 5. Potency, Efficacy, and Pharmacokinetic Properties of Modified C-3 Side Chain Derivatives. J. Med. Chem. 2000, 43, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Yang, W.; Wang, J.; Lin, L.; Wang, B. Synthesis and Evaluation of Dual Wavelength Fluorescent Benzo[b]thiophene Boronic Acid Derivatives for Sugar Sensing. Chem. Biol. Drug Des. 2007, 70, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Alagarsamy, V.; Prakash, C.R.; Selvam, T.P.; Karthick, V.; Kumar, P.D. Synthesis and anti-microbial activities of some 3-(4-phenylthiazole-2-yl)/3-(3-carbethoxy-4,5,6,7-tetrahydrobenzothiophene-2yl)quinazolin-4(3H)-one. RASĀYAN J. Chem. 2009, 2, 746–752. [Google Scholar]
- Starčević, K.; Kralj, M.; Piantanida, I.; Šuman, L.; Pavelić, K.; Karminski-Zamola, G. Synthesis, photochemical synthesis, DNA binding and antitumor evaluation of novel cyano- and amidino-substituted derivatives of naphtho-furans, naphtho-thiophenes, thieno-benzofurans, benzo-dithiophenes and their acyclic precursors. Eur. J. Med. Chem. 2006, 41, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Carballo, M.; Conde, M.; Tejedo, J.; Gualberto, A.; Jimenez, J.; Monteseirín, J.; Santa María, C.; Bedoya, F.J.; Hunt, S.W., III; Pintado, E.; et al. Macrophage inducible nitric oxide synthase gene expression is blocked by a benzothiophene derivative with anti-HIV properties. Mol. Genet. Metab. 2002, 75, 360–368. [Google Scholar]
- Batista, R.M.F.; Oliveira, R.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Ion Sensing Properties of New Colorimetric and Fluorimetric Chemosensors Based on Bithienyl-Imidazo-Anthraquinone Chromophores. Org. Lett. 2007, 9, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.M.F.; Oliveira, R.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Evaluation of bipendant-armed (oligo)thiophene crown ether derivatives as new chemical sensors. Tetrahedron Lett. 2008, 49, 6575–6578. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.F.; Oliveira, R.; Nuñez, C.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Evaluation of new thienyl and bithienyl-bis-indolymethanes as colorimetric sensors for anions. J. Phys. Org. Chem. 2009, 22, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.; Baptista, R.; Costa, S.P.G.; Raposo, M.M.M.; Lodeiro, C. Exploring the Emissive Properties of New Azacrown Compounds Bearing Aryl, Furyl and Thienyl Moieties: A Special case of Chelation Enhancement of Fluorescence upon interaction with Ca(II), Cu(II) or Ni(II). Inorg. Chem. 2010, 49, 10478–10857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, E.; Genovese, D.; Juris, R.; Zaccheroni, N.; Capelo, J.L.; Raposo, M.M.M.; Costa, S.P.G.; Prodi, L.; Lodeiro, C. Bioinspired systems for Metal-ion sensing: New emissive peptide probes based on benzo[d]oxazole derivatives and their gold and silica nanoparticles. Inorg. Chem. 2011, 50, 8834–8849. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Nunes-Miranda, J.D.; Santos, H.M. From colorimetric chemosensors to metal nanoparticles using two new tyrosine Schiff-base ligands for Cu(II) detection. Inorg. Chim. Acta 2012, 380, 22–30. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Núñez, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Bértolo, E.; Capelo, J.L.; Lodeiro, C. N1-(Benzo[b]thiophen-3-ylmethylidene)-N2-(2-((2-((2-(benzo[b]thiophen-3-ylmethylidene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine. Molbank 2012, 2012, M768. https://doi.org/10.3390/M768
Núñez C, Fernández-Lodeiro J, Fernández-Lodeiro A, Bértolo E, Capelo JL, Lodeiro C. N1-(Benzo[b]thiophen-3-ylmethylidene)-N2-(2-((2-((2-(benzo[b]thiophen-3-ylmethylidene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine. Molbank. 2012; 2012(3):M768. https://doi.org/10.3390/M768
Chicago/Turabian StyleNúñez, Cristina, Javier Fernández-Lodeiro, Adrián Fernández-Lodeiro, Emília Bértolo, José Luis Capelo, and Carlos Lodeiro. 2012. "N1-(Benzo[b]thiophen-3-ylmethylidene)-N2-(2-((2-((2-(benzo[b]thiophen-3-ylmethylidene)amino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine" Molbank 2012, no. 3: M768. https://doi.org/10.3390/M768






