3-(4-Fluorophenyl)-N-[4-(4-furan-2-yl-1-oxo-2,3,7-triazaspiro[4.5]dec-3-en-2-yl)phenyl]propionamide Hydrochloride
Abstract
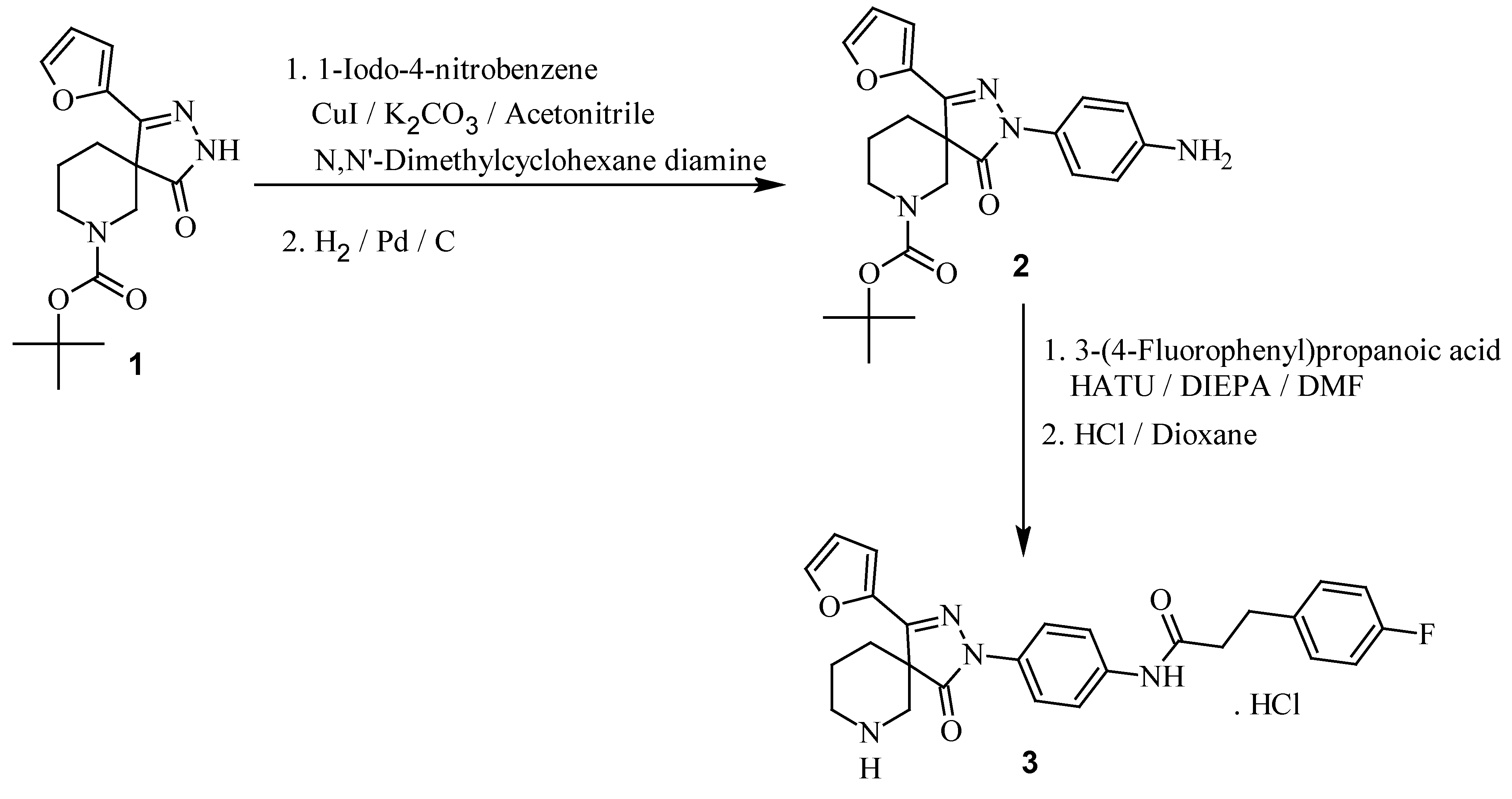
:Introduction
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References
- Mead, K.T.; Brewer, B.N. Strategies in spiro ketal synthesis revisited: Recent applications and advances. Curr. Org. Chem. 2003, 7, 227–256. [Google Scholar] [CrossRef]
- Shi, Z.-J.; Zhang, S.-L.; Cao, W.-G.; Deng, H.-M. Facile synthesis of a series of perfluoroalkyl-containing tetra-spirocyclic compounds and their spectral analysis. Chin. J. Chem. 2008, 26, 2103–2106. [Google Scholar] [CrossRef]
- Zhang, Z.-H. Synthesis and application of chiral spiro ligands in asymmetric catalysis. Chin. J. Org. Chem. 2005, 25, 355–363. [Google Scholar]
- Arai, M.A.; Kuraishi, M.; Arai, T.; Sasai, H. A new asymmetric Wacker-type cyclization and tandem cyclization promoted by Pd(II)-spiro bis(isoxazoline) catalyst. J. Am. Chem. Soc. 2001, 123, 2907–2908. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y.; Wang, Y.; William, J.B. The investigation of polymerization mechanism of 7-methylene-2-methyl-1,4,6-triox-aspiro(4,4)nonane. Acta Polym. Sin. 1989, 1, 18–24. [Google Scholar]
- Srinivasan, R.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. tert-Butyl 1-(furan-2-yl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-7-carboxylate. Molbank 2012, 2012, M757. [Google Scholar] [CrossRef]
- Srinivasan, R.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. N-(3,4-Dimethoxyphenyl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-1-carboxamide hydrochloride. Molbank 2012, 2012, M769. [Google Scholar] [CrossRef]
- Klapars, A.; Huang, X.; Buchwald, S. A general and efficient copper catalyst for the amidation of aryl halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar]
- Han, G.; Tamaki, M.; Hruby, V.J. Fast, efficient and selective deprotection of the tert-butoxycarbonyl (Boc) group using HCl/dioxane (4 M). J. Pept. Res. 2001, 58, 338–341. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Srinivasan, R.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. 3-(4-Fluorophenyl)-N-[4-(4-furan-2-yl-1-oxo-2,3,7-triazaspiro[4.5]dec-3-en-2-yl)phenyl]propionamide Hydrochloride. Molbank 2012, 2012, M771. https://doi.org/10.3390/M771
Srinivasan R, Narayana B, Samshuddin S, Sarojini BK. 3-(4-Fluorophenyl)-N-[4-(4-furan-2-yl-1-oxo-2,3,7-triazaspiro[4.5]dec-3-en-2-yl)phenyl]propionamide Hydrochloride. Molbank. 2012; 2012(3):M771. https://doi.org/10.3390/M771
Chicago/Turabian StyleSrinivasan, Rajagopalan, Badiadka Narayana, Seranthimata Samshuddin, and Balladka Kunhanna Sarojini. 2012. "3-(4-Fluorophenyl)-N-[4-(4-furan-2-yl-1-oxo-2,3,7-triazaspiro[4.5]dec-3-en-2-yl)phenyl]propionamide Hydrochloride" Molbank 2012, no. 3: M771. https://doi.org/10.3390/M771




