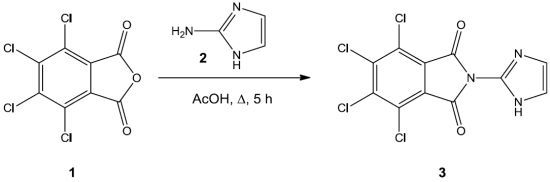
4,5,6,7-Tetrachloro-2-(1H-imidazol-2-yl)isoindoline-1,3-dione
Abstract
:Experimental
General
4,5,6,7-Tetrachloro-2-(1H-imidazol-2-yl)isoindoline-1,3-dione (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Miyachi, H.; Azuma, A.; Ogasawara, A.; Uchimura, E.; Watanabe, N.; Kobayashi, Y.; Kato, F.; Kato, M.; Hashimoto, Y. Novel biological response modifiers: Phthalimides with tumor necrosis factor-α production-regulating activity. J. Med. Chem. 1997, 40, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Sou, S.; Mayumi, S.; Takahashi, H.; Yamasaki, R.; Kadoya, S.; Sodeoka, M.; Hashimoto, Y. Novel α-glucosidase inhibitors with a tetrachlorophthalimide skeleton. Bioorg. Med. Chem. Lett. 2000, 10, 1081–1084. [Google Scholar] [CrossRef]
- Motoshima, K.; Noguchi-Yachide, T.; Sugita, K.; Hashimoto, Y.; Ishikawa, M. Separation of α-glucosidase-inhibitory and liver X receptor-antagonistic activities of phenethylphenyl phthalimide analogs and generation of LXRα-selective antagonists. Bioorg. Med. Chem. 2009, 17, 5001–5014. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.-M.; El-Azab, A.S.; Attia, S.M.; Al-Obaid, A.M.; Al-Omar, M.A.; El-Subbagh, H.I. Synthesis and biological evaluation of some novel cyclic-imides as hypoglycaemic, anti-hyperlipidemic agents. Eur. J. Med. Chem. 2011, 46, 4324–4329. [Google Scholar] [CrossRef] [PubMed]
- Golub, A.G.; Yakovenko, O.Y.; Prykhod'ko, A.O.; Lukashov, S.S.; Bdzhola, V.G.; Yarmoluk, S.M. Evaluation of 4,5,6,7-tetrahalogeno-1H-isoindole-1,3(2H)-diones as inhibitors of human protein kinase CK2. Biochem. Biophys. Acta Protein Proteonomics 2008, 1784, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Egert-Schmidt, A.-M.; Dreher, J.; Dunkel, U.; Kohfeld, S.; Preu, L.; Weber, H.; Ehlert, J.E.; Mutschler, B.; Totzke, F.; Schächtele, C.; et al. Identification of 2-anilino-9-methoxy-5,7-dihydro-6H-pyrimido[5,4-d][1]benzazepin-6-ones as dual PLK1/VEGF-R2 kinase inhibitor chemotypes by structure-based lead generation. J. Med. Chem. 2010, 53, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.F.; Pattabiraman, N.; Kellogg, G.E.; Lemcke, T.; Kunick, C.; Sausville, E.A.; Zaharevitz, D.W.; Gussio, R. Homology model of the CDK1/cyclin B complex. J. Biomol. Struct. Dyn. 2005, 22, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tolle, N.; Dunkel, U.; Oehninger, L.; Ott, I.; Preu, L.; Haase, T.; Behrends, S.; Jones, P.G.; Totzke, F.; Schächtele, C.; et al. Synthesis and structure of fluorescent chelate boron complexes of 4-anilinomethylidene-1-benzazepine-2,5-dione ligands. Synthesis 2011, 2848–2858. [Google Scholar]
- Xie, X.; Lemcke, T.; Gussio, R.; Zaharevitz, D.W.; Leost, M.; Meijer, L.; Kunick, C. Epoxide-containing side chains enhance antiproliferative activity of paullones. Eur. J. Med. Chem. 2005, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, H.; Harre, M.; Koenig, K.; Merten, E.; Tilstam, U. Efficient and environmentally friendly synthesis of 2-amino-imidazole. Tetrahedron Lett. 2002, 43, 593–595. [Google Scholar] [CrossRef]
- Voegel, J.J.; Altorfer, M.M.; Benner, S.A. The donor-acceptor-acceptor purine analog: Transformation of 5-aza-7-deaza-1H-isoguanine (=4-aminoimidazo[1,2-a]-1,3,5-triazin-2(1H)-one) to 2'-deoxy-5-aza-7-deaza-isoguanosine using purine nucleoside phosphorylase. Helv. Chim. Acta 1993, 76, 2061–2069. [Google Scholar] [CrossRef]
- Pratt, D.S.; Young, C.O. Phthalic acid derivatives: Constitution and color. XIV. Some derivatives of tetrabromophthalimide. J. Am. Chem. Soc. 1918, 40, 1415–1425. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wölfel, S.; Berndt, F.; Friedrichs, J.; Haese, M.; Joostberends, J.; Masri, B.; Schnerre, R.; Wabnik, M.; Kunick, C. 4,5,6,7-Tetrachloro-2-(1H-imidazol-2-yl)isoindoline-1,3-dione. Molbank 2012, 2012, M785. https://doi.org/10.3390/M785
Wölfel S, Berndt F, Friedrichs J, Haese M, Joostberends J, Masri B, Schnerre R, Wabnik M, Kunick C. 4,5,6,7-Tetrachloro-2-(1H-imidazol-2-yl)isoindoline-1,3-dione. Molbank. 2012; 2012(4):M785. https://doi.org/10.3390/M785
Chicago/Turabian StyleWölfel, Sebastian, Frederik Berndt, Jessica Friedrichs, Michael Haese, Janine Joostberends, Bader Masri, Renate Schnerre, Maximilian Wabnik, and Conrad Kunick. 2012. "4,5,6,7-Tetrachloro-2-(1H-imidazol-2-yl)isoindoline-1,3-dione" Molbank 2012, no. 4: M785. https://doi.org/10.3390/M785