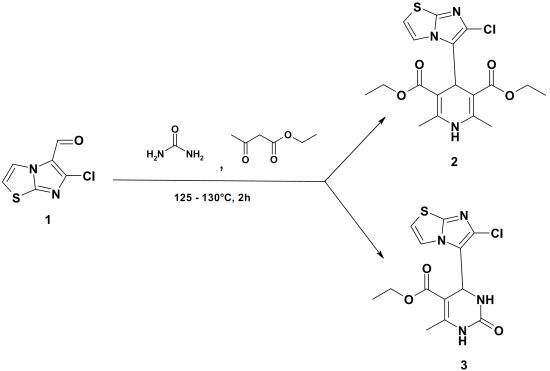
Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate and Ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Abstract
:
Experimental
General
Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (2), Ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (3)
Data for Compound 2
Data for Compound 3
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Biginelli, P. Intorno ad uramidi aldeidiche dell’etere acetilacetico. Gazz. Chim. Ital. 1891, 497–500. [Google Scholar]
- Suresh; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. Arkivoc 2012, i, 66–133. [Google Scholar]
- Kastron, V.V.; Vitolin, R.O.; Khanina, E.L.; ya Duburs, G.; Kimenis, A.A. Fluorine-containing derivatives of 1,2,3,4-tetrahydropyrimidine: Synthesis and pharmacological activity. Khim. Farm. Zh. 1987, 21, 948–952. [Google Scholar] [CrossRef]
- Trivedi, A.R.; Bhuva, V.R.; Dholariya, B.H.; Dodiya, D.K.; Kataria, V.B.; Shah, V.H. Novel dihydropyrimidines as a potential new class of antitubercular agents. Bioorg. Med. Chem. Lett. 2010, 20, 6100–6102. [Google Scholar] [CrossRef] [PubMed]
- Budriesi, R.; Ioan, P.; Locatelli, A.; Leoni, A.; Cosconati, S.; di Toro, R.; Chiarini, A.; Spampinato, S.; Marinelli, L.; Novellino, E.; et al. Imidazo[2,1-b]thiazole system: A scaffold endowning dihydropyridines with selective cardiopressant activity. J. Med. Chem. 2008, 51, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Hajra, A.; Dey, S.S. A practical and green approach towards synthesis of Dihydropyrimidinones without any solvent or catalyst. Org. Process Res. Dev. 2002, 6, 817–818. [Google Scholar] [CrossRef]
- Andreani, A.; Rambaldi, M.; Locatelli, A.; Andreani, F. 5-Formylimidazo[2,1-b]thiazoles and derivatives with herbidal acivity. Collect. Czech. Chem. Commun. 1991, 56, 2436–2437. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morigi, R.; Locatelli, A.; Rambaldi, M.; Consorti, A.; Leoni, A. Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate and Ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2012, 2012, M787. https://doi.org/10.3390/M787
Morigi R, Locatelli A, Rambaldi M, Consorti A, Leoni A. Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate and Ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank. 2012; 2012(4):M787. https://doi.org/10.3390/M787
Chicago/Turabian StyleMorigi, Rita, Alessandra Locatelli, Mirella Rambaldi, Alessandro Consorti, and Alberto Leoni. 2012. "Diethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate and Ethyl 4-(6-Chloroimidazo[2,1-b]thiazol-5-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate" Molbank 2012, no. 4: M787. https://doi.org/10.3390/M787