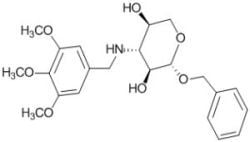
Benzyl 3-deoxy-3-(3,4,5-trimethoxybenzylamino)-β-L-xylopyranoside
Abstract
:Results and Discussion
Experimental
General
| C22H29NO7 | |
| Calculated: | C 62.99; H, 6.97; N, 3.34; O, 26.70 |
| Found: | C 62.89; H, 7.01; N, 3.29; O, 26.65 |
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Lugemwa, F.N.; Sarkar, A.; Esko, J.D. Unusual β-d-xylosides that prime glycosaminoglycan in animal cells. J. Biol. Chem. 1996, 271, 19159–19165. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chang, J.-Y.; Nien, C.-Y.; Kuo, C.-C.; Shih, K.-H.; Wu, C.-H.; Chang, C.-Y.; Lai, W.-Y.; Liou, J.-P. 5-Amino-2 aroylquinolines as highly potent tubulin polymerization inhibitor. Part 2. The impact of bridging groups at position C-2. J. Med. Chem. 2011, 54, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Denny, W.A.; Tercel, M.; Pruijn, F.B.; Ashoorzadeh, A. Nitro seco analogues of the duocarmycins containing sulfonate leaving groups as hypoxia-activated prodrugs for cancer therapy. J. Med. Chem. 2012, 55, 2780–2802. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Luo, J.; Yang, F.; Liu, T.; Liu, M.; Qiu, W.-W.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-fused betulinic acid derivatives as novel inhibitors of osteoclast differentiation and bone resorption. J. Med. Chem. 2012, 55, 3122–3134. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.C.; Whittington, D.A.; Doherty, E.M.; Falsey, J.R.; Chend, A.C.; Emkey, R.; Brake, R.L.; Lewis, R.T. Rapid development of piperidone carboxamides as potent and selective anaplastic lymphoma kinase inhibitors. J. Med. Chem. 2012, 55, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Solinas, A.; Faure, H.; Roudaut, H.; Traiffort, E.; Schoenfelder, A.; Mann, A.; Manetti, F.; Taddei, M.; Ruat, M. Acylthiourea, acylurea, and acylguanidine derivatives with potent hedgehog inhibiting activity. J. Med. Chem. 2012, 55, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, F.; Pagliarin, R.; Fumagalli, G.; Bigi, A.; Fusi, P.; Orsini, F.; Frattini, M.; Coccetti, P. Synthesis and biological evaluation of 1,4-diaryl-2-azetidinones as specific anticancer Agents: Activation of adenosine monophosphate activated protein kinase and induction of apoptosis. J. Med. Chem. 2012, 55, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Pellicani, R.Z.; Stefanachi, A.; Niso, M.; Carotti, A.; Leonetti, F.; Nicolotti, O.; Perrone, R.; Berardi, F.; Cellamare, S.; Colabufo, N.A. Potent galloyl-based selective modulators targeting multidrug resistance associated protein 1 and P-glycoprotein. J. Med. Chem. 2012, 55, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.L.; Gill, G.S.; Grobelny, D.W.; Chaplin, J.H.; Paul, D.; Leske, A.F.; Lavranos, T.C.; Chalmers, D.K.; Charman, S.A.; Kostewicz, E.; et al. Discovery of 7-hydroxyl-6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzo[b]furan (BNC105), a tubulin polymerization inhibitor with potent antiproliferative and tumor vascular disrupting properties. J. Med. Chem. 2011, 54, 6014–6027. [Google Scholar] [CrossRef] [PubMed]
- Theeramunkong, S.; Caldarelli, A.; Massarotti, A.; Aprile, S.; Caprioglio, D.; Zaninetti, R.; Teruggi, A.; Pirali, T.; Grosa, G.; Tron, G.C.; et al. Regioselective Suzuki coupling of dihaloheteroaromatic compounds as a rapid strategy to synthesize potent rigid combretastation analogues. J. Med. Chem. 2011, 54, 4977–4986. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Brancale, A.; Ricci, A.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. Convergent synthesis and biological evaluation of 2-amino-4(3′,4′,5′-trimethoxyphenyl)-5-aryl thiazoles as microtubule targeting agents. J. Med. Chem. 2011, 54, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, C.-M.; Wang, Z.; Chen, J.; Mohler, M.L.; Li, W.; Dalton, J.T.; Miller, D.D. Design, synthesis, and SAR studies of 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents. J. Med. Chem. 2011, 54, 4678–4693. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.; Barbier, P.; Douillard, S.; McLeer-Florin, A.; Bourgarel-Rey, V.; Pierson, J.-T.; Fedorov, A.Y.; Finet, J.-P.; Boutonnat, J.; Peyrot, V. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. Part 2. J. Med. Chem. 2011, 54, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Lugemwa, F.N.; Denison, L. Synthesis of 3-substituted xylopyranosides from 2,3-anhydropentosides. J. Carbohydr. Chem. 1997, 16, 1433–1441. [Google Scholar] [CrossRef]
- Lugemwa, F.N.; Denison, L. Benzyl 3-deoxy-3-(phenethylamino) β-l-xylopyranoside. Molecules 1998, 3, M76. [Google Scholar] [CrossRef]

© 2013 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lugemwa, F.N. Benzyl 3-deoxy-3-(3,4,5-trimethoxybenzylamino)-β-L-xylopyranoside. Molbank 2013, 2013, M793. https://doi.org/10.3390/M793
Lugemwa FN. Benzyl 3-deoxy-3-(3,4,5-trimethoxybenzylamino)-β-L-xylopyranoside. Molbank. 2013; 2013(1):M793. https://doi.org/10.3390/M793
Chicago/Turabian StyleLugemwa, Fulgentius Nelson. 2013. "Benzyl 3-deoxy-3-(3,4,5-trimethoxybenzylamino)-β-L-xylopyranoside" Molbank 2013, no. 1: M793. https://doi.org/10.3390/M793