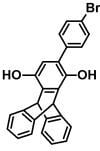
4'-Bromophenyl Triptycene-2,5-diol
Abstract
:Experimental
Step 1: Diel’s Alder Reaction
Step 2: Reduction
Br-PQ
T-Br-PH
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Liu, B.; Robertson, G.P.; Guiver, M.D.; Shi, Z.; Navessin, T.; Holdcroft, S. Fluorinated poly(aryl ether) containing a 4-bromophenyl pendant group and its phosphonated derivative. Macromol. Rapid Commun. 2006, 27, 1411–1417. [Google Scholar] [CrossRef]
- Alonso, M.Á.; Alvarado, P.L.; Avendaño, C.; Menéndez, C.J. Regioselective Diels-Alder reactions of 3-vinylindoles with quinones. Lett. Org. Chem. 2004, 1, 20–22. [Google Scholar] [CrossRef]
- Taylor, M.S.; Swager, T.M. Triptycenediols by rhodium-catalyzed [2+2+2] cycloaddition. Org. Lett. 2007, 9, 3695–3697. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W., III; Long, T.M.; Pate, B.D.; Kline, S.R.; Thomas, E.L.; Swager, T.M. Perpendicular organization of macromolecules: Synthesis and alignment studies of a soluble poly(iptycene). J. Am. Chem. Soc. 2005, 127, 17976–17977. [Google Scholar] [CrossRef] [PubMed]
- Long, T.M.; Swager, T.M. Triptycene-containing bis(phenylethynyl)benzene nematic liquid crystals. J. Mater. Chem. 2002, 12, 3407–3412. [Google Scholar] [CrossRef]
- Song, M.H.; Park, B.; Nishimura, S.; Toyooka, T.; Chung, I.J.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Electrotunable non-reciprocal laser emission from a liquid-crystal photonic device. Adv. Funct. Mater. 2006, 16, 1793–1798. [Google Scholar] [CrossRef]
- Chmiel, J.; Heesemann, I.; Mix, A.; Neumann, B.; Stammler, H.G.; Mitzel, N.W. The effect of bulky substituents on the formation of symmetrically trisubstituted triptycenes. Eur. J. Org. Chem. 2010, 20, 3897–3907. [Google Scholar] [CrossRef]
- Swager, T.M. Iptycenes in the design of high performance polymers. Acc. Chem. Res. 2008, 41, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Z.; Wirth, T. Triptycene derivatives: Synthesis and applications. Chem. Lett. 2010, 39, 658–661. [Google Scholar] [CrossRef]
- Vacek, J.; Michl, J. Artificial surface-mounted molecular rotors: Molecular dynamics simulations. Adv. Funct. Mater. 2007, 17, 730–739. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 67–69. [Google Scholar] [CrossRef] [PubMed]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ganesh, S.D.; Pai, K.V.; Karidurganavar, M.Y. 4'-Bromophenyl Triptycene-2,5-diol. Molbank 2013, 2013, M796. https://doi.org/10.3390/M796
Ganesh SD, Pai KV, Karidurganavar MY. 4'-Bromophenyl Triptycene-2,5-diol. Molbank. 2013; 2013(1):M796. https://doi.org/10.3390/M796
Chicago/Turabian StyleGanesh, Shimoga D., Karkala V. Pai, and Mahadevappa Y. Karidurganavar. 2013. "4'-Bromophenyl Triptycene-2,5-diol" Molbank 2013, no. 1: M796. https://doi.org/10.3390/M796