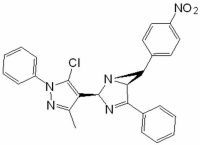
2-(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene
Abstract
:Introduction
Results and Discussion
Experimental
Preparation of 3-Methyl-1-phenyl-1H-pyrazol-5(4H)-one (6)
Preparation of 5-Chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde (3)
Synthesis of 2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene (7a)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflict of Interest
References
- Ishibashi, Y.; Fujiwara, M.; Umesato, T.; Saito, H.; Kobatake, S.; Irie, M.; Miyasaka, H. Cyclization Reaction Dynamics of a Photochromic Diarylethene Derivative as Revealed by Femtosecond to Microsecond Time-Resolved Spectroscopy. J. Phys. Chem. C 2011, 115, 4265–4272. [Google Scholar] [CrossRef]
- Yun, C.; You, J.; kim, J.; Huh, J.; Kim, E. Photochromic fluorescence switching from diarylethenes and its applications. J. Photoch. Photobiol. C Photochem. Rev. 2009, 10, 111–129. [Google Scholar] [CrossRef]
- Ashraf, M.; Gainsford, G.J.; Kay, A.J. Crystal and Molecular Structure of 7-Diethylamino-[1',3',3'-trimethyl-4-((1,3,3-trimethyl-1,3-dihydro-2H-indole-2-ylidene)methyl)-1',3,3',4-tetrahydrospiro[chromene-2,2'-indole]]: A Dicondensation Product From the Reaction of 4-Diethylaminosalicylaldehyde with Fischer’s Base. Aust. J. Chem. 2012, 65, 779–784. [Google Scholar]
- Patel, P.D.; Masunov, A.E. Theoretical study of photochromic compounds: Part 3. Prediction of thermal stability. J. Phys. Chem. C 2011, 115, 10292–10297. [Google Scholar] [CrossRef]
- Patel, P.D.; Masunov, A.E. Theoretical study of photochromic compounds. 1. Bond length alternation and absorption spectra for the open and closed forms of 29 diarylethene derivatives. J. Phys. Chem. A 2009, 113, 8409–8414. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, D.; Perpete, E.A.; Maurel, F.; Perrier, A. Doubly closing or not? Theoretical analysis for coupled photochromes. J. Phys. Chem. C 2010, 114, 9489–9497. [Google Scholar] [CrossRef]
- Crano, J.C.; Flood, T.; Knowles, D.; Kumar, A.; Van Gemert, B. Photochromic compounds: Chemistry and application in ophthalmic lenses. Pure Appl. Chem. 1996, 68, 1395–1398. [Google Scholar] [CrossRef]
- Dvornikov, A.S.; Malkin, J.; Rentzepis, P.M. Spectroscopy and kinetics of photochromic materials for 3D optical memory devices. J. Phys. Chem. 1994, 98, 6746–6752. [Google Scholar] [CrossRef]
- Willner, I.; Rubin, S.; Shatzmiller, R.; Zor, T. Reversible light-stimulated activation and deactivation of .alpha.-chymotrypsin by its immobilization in photoisomerizable copolymers. J. Am. Chem. Soc. 1993, 115, 8690–8694. [Google Scholar] [CrossRef]
- Winkler, J.D.; Deshayes, K.; Shao, B. Photodynamic transport of metal ions. J. Am. Chem. Soc. 1989, 111, 769–770. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Mamaghani, M.; Behzadi, T. Synthesis and structure–behavior relationships of tetra-substituted imidazole derivatives of 1,3-diazabicyclo[3,1,0]hex-3-ene. Mol. Divers. 2012, 16, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Besharati-Seidani, T.; Mahmoodi, N.O. Synthesis of new bicyclic aziridines containing chalcone analogs and investigation of their photochromic properties. Bull. Korean Chem. Soc. 2013, 34, 875–883. [Google Scholar] [CrossRef]
- Kiyani, H.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Photochromic behavior of several new synthesized Bis-1,3-Diazabicyclo[3.1.0]hex-3-enes. J. Phys. Org. Chem. 2009, 22, 559–567. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Kiyani, H.; Yazdanbakhsh, M.R.; Sharifzadeh, B. Synthesis and photochromic properties of new heterocyclic derivatives of 1,3-diazabicyclo[3.1.0]hex-3-ene. J. Chin. Chem. Soc. 2007, 54, 635–641. [Google Scholar] [CrossRef]
- Kiyani, H.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Synthesis and photochromism of 1,3-diazabicyclo[3.1.0]hex-3-ene Phenol Rings. Mendeleev. Commun. 2009, 19, 203–205. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Tabatabaeian, K.; Kiyani, H. Two 1,3-diazabicyclo[3.1.0]hex-3-enes with a ‘Tripod’ core. Helv. Chim. Acta 2012, 95, 536–542. [Google Scholar] [CrossRef]
- Dyakonenko, V.V.; Maleev, A.V.; Zbruyev, A.I.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V. Layered crystal structure of bicyclic aziridines as revealed by analysis of intermolecular interactions energy. Cryst. Eng. Comm. 2010, 12, 1816–1823. [Google Scholar] [CrossRef]
- Bruno, G.; Nicol, F.; Rotondo, A.; Risitano, F.; Grassi, G.; Foti, F. Structure investigation of bridgehead aziridine: Synthesis, theoretical, and crystallographic study of 2,4,6-Triphenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Helv. Chim. Acta 2006, 89, 190–200. [Google Scholar] [CrossRef]
- Risitano, F.; Grassi, G.; Foti, F.; Moraci, S. A novel efficient three-component one-pot synthesis of 1,3-diazabicyclo[3.1.0]hex-3-ene system under microwave irradiation. Synlett 2005, 1633–1635. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Tabatabaeian, K.; Ghavidast, A. Synthesis and photochromic behavior of mono, and biphotochromic system linked by p-Phenylene bridge. Chin. Chem. Lett. 2010, 21, 1199–1202. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Desenko, S.M.; Gurley, T.W. Azaheterocycles Based on α,β-Unsaturated Carbonyls; Springer-Verlag: Berlin&Heidelberg, Germany, 2008; Chapter 1; pp. 17–32. [Google Scholar]
- Trozolo, A.M.; Leslie, T.M.; Sarportdar, A.S.; Small, R.D.; Ferraudi, G.J.; DoMinh, T.; Hartless, R.L. Photochemistry of Some Three-Membered Heterocycles. Pure Appl. Chem. 1979, 51, 261–270. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Kiyani, H. Synthesis of Thiophene Derivatives of 1,3-Diazabicyclo[3,1,0]hex-3-ene. Bull. Korean Chem. Soc. 2004, 25, 1417–1420. [Google Scholar]
- Dürr, H. Perspectives in Photochromism: A Novel System Based on the 1,5-Electrocyclization of Heteroanalogous Pentadienyl Anions. Angew. Chem. Int. Ed. Engl. 1989, 28, 413–431. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Marvidis, I.M. Photochromism and Thermochromism of Schiff Bases in the Solid State: Structural Aspects. Chem. Soc. Rev. 2004, 33, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hiene, H.W.; Weese, R.H.; Cooper, R.A.; Aziridines, X.V. The synthesis and reactions of 1,3-diazabicyclo[3.1.0]hex-3-enes. J. Org. Chem. 1967, 32, 2708–2711. [Google Scholar] [CrossRef]
- Padwa, A.; Glazer, E. Photochemical reorganizations in the 1,3-diazabicyclo[3.1.0]hex-3-ene system. J. Am. Chem. Soc. 1972, 94, 7788–7797. [Google Scholar] [CrossRef]
- Padwa, A.; Clough, S.; Glazer, E. Photochemical transformations of small-ring heterocyclic compounds. XXIV. Photoisomerization of the triphenyl-1,3-diazabicyclo[3.1.0]hex-3-ene system. J. Am. Chem. Soc. 1970, 92, 1778–1779. [Google Scholar] [CrossRef]
- Dyakonenko, V.V.; Shishkin, O.V.; Zbruev, A.V.; Desenko, S.M. 2,2-Dimethyl-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Acta Crystallogr. Sect. E: Struct. Rep. Online 2005, E61, o667–o668. [Google Scholar] [CrossRef]
- Zbruyev, A.I.; Vashchenko, V.V.; Andryushchenko, A.A.; Desenko, S.M.; Musatov, V.I.; Knyazeva, I.V.; Chebanov, V.A. Synthesis of polyarene derivatives of fused aziridines by Suzuki-Miyaura cross-coupling. Tetrahedron 2007, 63, 4297–4303. [Google Scholar] [CrossRef]
- Kiyani, H. 2-(4-Diethoxymethylphenyl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Molbank 2012, 2012, M780. [Google Scholar] [CrossRef]
- Kiyani, H.; Ardyanian, M. N-(4-(6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene-2-yl)phenyl)acetamide. Molbank 2013, 2013, M791. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S. Fused Aziridines as Sources of Azomethine ylides. Chem. Heterocyl. Compd. 2012, 48, 179–190. [Google Scholar] [CrossRef]
- D’yakonenko, V.V.; Zbruev, A.V.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V. Molecular and Crystal Structure of 3,3-Dimethyl-5-(2-naphthyl)-1-(4-nitrophenyl)-3,5a-dihydro-1H-azireno[1,2-c]imidazole. J. Struct. Chem. 2005, 46, 1110–1113. [Google Scholar] [CrossRef]
- Zbruev, A.I.; Panikarskaya, V.D.; Kasyan, N.A.; Zavora, L.N.; Lisetskii, L.N.; Desenko, S.M.; Chebanov, V.A. The photoinduced transformations of aziridine derivatives in liquid crystalline matrices. Russ. J. Phys. Chem. A 2009, 83, 1350–1354. [Google Scholar] [CrossRef]
- Zbruev, A.I.; Yarmenko, F.G.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V.; Lukinova, E.V.; Knyazeva, I.V. Synthesis and study of new 2-aryl-1-(4-nitrophenyl)-1,1a-dihydroazireno[1,2-a]quinoxaline derivatives. Russ. Chem. Bull. 2006, 55, 362–368. [Google Scholar] [CrossRef]
- Kaluski, Z.; Figas, E.; Vorobyeva, N.P.; Orlov, V.D. Crystal and molecular structure of 2-(4-methoxyphenyl)-4-phenyl-6-(4-nitrophenyl)-1,3-diazabicylo[3.1.0]hex-3-ene. J. Struct. Chem. 1994, 35, 134–137. [Google Scholar] [CrossRef]
- Ragab, F.A.; Abdel Gawad, N.M.; Georgey, H.H.; Said, M.F. Synthesis of novel 1,3,4-trisubstituted pyrazoles as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2013, 63, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Damlijanovic, I.; Vukicevic, M.; Radulovic, N.; Palic, R.; Ellmerer, E.; Ratkovic, Z.; Joksovic, M.D.; Vukicevic, R.D. Synthesis and antimicrobial activity of some new pyrazole derivatives containing a ferrocene unit. Bioorg. Med. Chem. Lett. 2009, 19, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Rajpara, K.M.; Joshi, V.V. Synthesis of pyrazole encompassing 2-pyridone derivatives as antibacterial agents. Bioorg. Med. Chem. Lett. 2013, 23, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Amnerkar, N.D.; Bhusari, K.P. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur. J. Med. Chem. 2010, 45, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Clapham, K.M.; Batsanov, A.S.; Bryce, M.R.; Tarbit, B. Trifluoromethyl-substituted pyridyl- and pyrazolylboronic acids and esters: Synthesis and Suzuki–Miyaura cross-coupling reactions. Org. Biomol. Chem. 2009, 7, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.-C.; Li, H.-Q.; Sun, J.; Zhou, Y.; Zhu, H.-L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2010, 18, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, Z.; Kandilici, H.B.; Gumusel, B.; Calis, U.; Bilgin, A. Synthesis and Studies on Antidepressant and Anticonvulsant Activities of Some 3-(2-furyl)-pyrazoline Derivatives. Eur. J. Med. Chem. 2007, 42, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bebernitz, G.; Argentiery, G.; Battle, B.; Brennan, C.; Balkan, B.; Byrkey, B.; Eckhardt, M.; Gao, J.; Kapa, P.; Strohschein, R.; et al. The effect of 1,3-diaryl-[1H]-pyrazole-4-acetamides on glucose utilization in ob/ob Mice. J. Med. Chem. 2001, 44, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, K.; Park, S.-J.; Ahn, B.; Lee, J.-C.; Cho, H.; Lee, K.-I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.; Kasim-Sener, M.; Bildmci, I.; Kasimogullari, R.; Akçamur, Y. Studies on the reactions of cyclic oxalyl compounds with hydrazines or hydrazones: Synthesis and reactions of 4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxylic acid. J. Heterocycl. Chem. 2002, 39, 869–875. [Google Scholar] [CrossRef]
- Rangaswamy, J.; Kumar, H.V.; Harini, S.T.; Naik, N. Synthesis of benzofuran based 1,3,5-substituted pyrazole derivatives: As a new class of potent antioxidants and antimicrobials-A novel accost to amend biocompatibility. Bioorg. Med. Chem. Lett. 2012, 22, 4773–4772. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.B.; Chovatia, P.T.; Parekh, H.H. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.B.; Romagnoli, C.; Andreotti, E.; Mares, D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J. Agric. Food Chem. 2007, 55, 10331–10338. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Shetty, P.; Sundershan, S.; Fun, H.K. New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur. J. Med. Chem. 2013, 62, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.Z.; Surga, W.J.; Opozda, E.M. Palladium (II) methylpyrazole complexes. Transition Met. Chem. 1994, 19, 353–354. [Google Scholar] [CrossRef]
- Pavani, K.; Singh, M.; Ramanan, A. Oxalate bridged copper pyrazole complex templated anderson-evans cluster based solids. Aust. J. Chem. 2011, 64, 68–76. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Kamath, A.; Budagumpi, S.; Revankar, V.K. Pyrazole bridged binuclear transition metal complexes: Synthesis, characterization, antimicrobial activity and DNA binding/cleavage studies. J. Mol. Struct. 2011, 1006, 580–588. [Google Scholar] [CrossRef]
- Xu, C.-J.; Shi, Y.-Q. Synthesis and crystal structure of 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde. J. Chem. Crystallogr. 2011, 41, 1816–1819. [Google Scholar] [CrossRef]



© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Albooye, F.; Kiyani, H. 2-(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Molbank 2013, 2013, M806. https://doi.org/10.3390/M806
Albooye F, Kiyani H. 2-(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Molbank. 2013; 2013(3):M806. https://doi.org/10.3390/M806
Chicago/Turabian StyleAlbooye, Fereshteh, and Hamzeh Kiyani. 2013. "2-(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene" Molbank 2013, no. 3: M806. https://doi.org/10.3390/M806