(Z)-4-(Carbomethoxymethylene)-2-(4-fluorophenyl)-4H-benzo[d][1,3]oxazine
Abstract
:1. Introduction
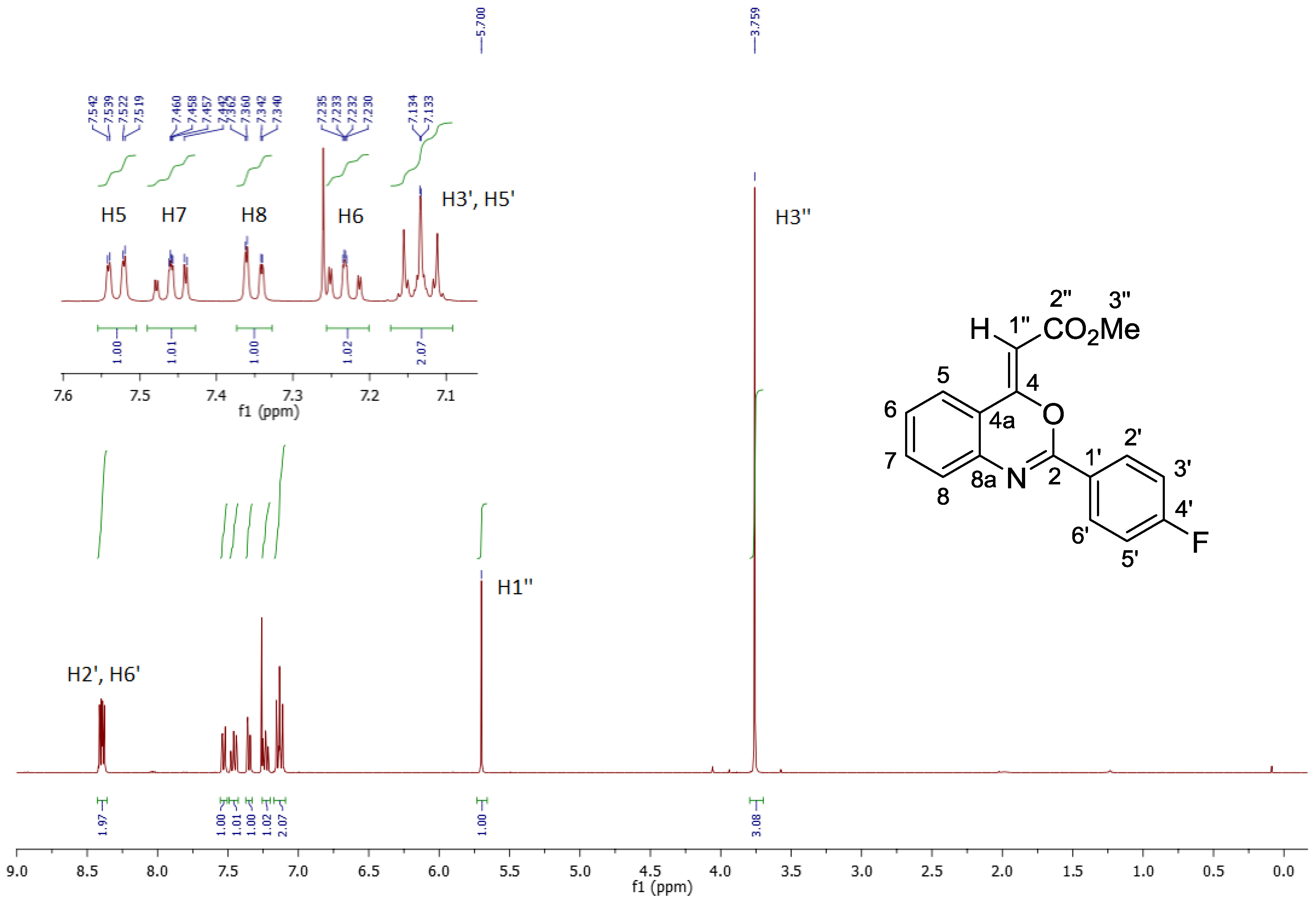
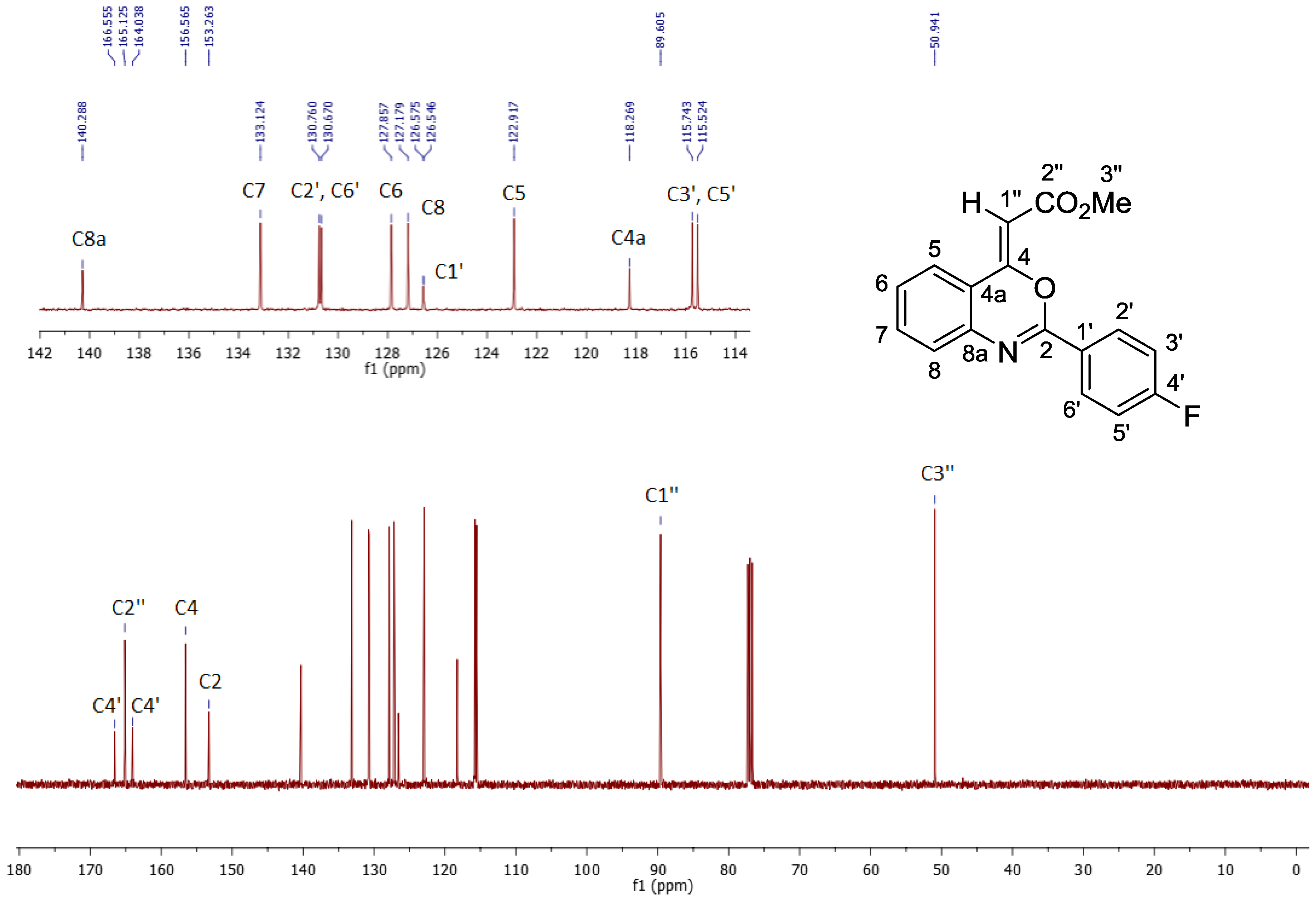
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gadge, S.; Bhanage, B. Recent developments in palladium catalysed carbonylation reactions. RSC Adv. 2013, 4, 10367–10389. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative Carbonylation as a Powerful Tool for the Direct Synthesis of Carbonylated Heterocycles. Eur. J. Org. Chem. 2012, 6825–6839. [Google Scholar] [CrossRef]
- Shen, C.; Wu, X. Palladium-Catalyzed Carbonylative Multicomponent Reactions. Chem. Eur. J. 2016, (in press). [Google Scholar] [CrossRef] [PubMed]
- Rossi, E. Palladium-Assisted Synthesis of Heterocycles via Carbonylation Reactions. In Modern Carbonylation Methods; Kollár, L., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 321–362. [Google Scholar]
- Bacchi, A.; Costa, M.; Della Ca’, N.; Fabbricatore, M.; Fazio, A.; Gabriele, B.; Nasi, C.; Salerno, G. Synthesis of 1 (Alkoxycarbonyl) methylene 1, 3 dihydroisobenzofurans and 4 (Alkoxycarbonyl) benzo [c] pyrans by Palladium Catalysed Oxidative Carbonylation of 2 Alkynylbenzyl Alcohols, 2 Alkynylbenzaldehydes and 2 Alkynylphenyl Ketones. Eur. J. Org. Chem. 2004, 574–585. [Google Scholar] [CrossRef]
- Bacchi, A.; Costa, M.; Della Ca’, N.; Gabriele, B.; Salerno, G.; Cassoni, S. Heterocyclic derivative syntheses by palladium-catalyzed oxidative cyclization-alkoxycarbonylation of substituted γ-oxoalkynes. J. Org. Chem. 2005, 70, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Campanini, F.; Gabriele, B.; Salerno, G.; Massera, C.; Costa, M. Cascade Reactions: Catalytic Synthesis of Functionalized 1,3-Dihydroisobenzofuran and Tetrahydrofuran Derivatives by Sequential Nucleophilic Ring Opening-Heterocyclization- Oxidative Carbonylation of Alkynyloxiranes. Adv. Synth. Catal. 2009, 351, 2423–2432. [Google Scholar] [CrossRef]
- Della Ca’, N.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-catalyzed synthesis of symmetrical urea derivatives by oxidative carbonylation of primary amines in carbon dioxide medium. J. Catal. 2011, 282, 120–127. [Google Scholar]
- Queirolo, M.; Vezzani, A.; Mancuso, R.; Gabriele, B.; Costa, M.; Della Ca’, N. Neutral vs anionic palladium iodide-catalyzed carbonylation of terminal arylacetylenes. J. Mol. Catal. A: Chem. 2015, 398, 115–126. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic Oxidative Carbonylation of Amino Moieties to Ureas, Oxamides, 2-Oxazolidinones, and Benzoxazolones. ChemSusChem 2015, 8, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Maner, A.; Ziccarelli, I.; Pomelli, C.; Chiappe, C.; Della Ca’, N.; Veltri, L.; Gabriele, B. Auto-Tandem Catalysis in Ionic Liquids: Synthesis of 2-Oxazolidinones by Palladium-Catalyzed Oxidative Carbonylation of Propargylic Amines in EmimEtSO4. Molecules 2016, 21, 897. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Giacoia, G.; Mancuso, R.; Gabriele, B.; Motti, E.; Costa, M.; Della Ca’, N. A highly efficient Pd/CuI-catalyzed oxidative alkoxycarbonylation of α-olefins to unsaturated esters. J. Mol. Catal. A: Chem. 2017, 426, 435–443. [Google Scholar] [CrossRef]
- Costa, M.; Della Ca’, N.; Gabriele, B.; Massera, C.; Salerno, G.; Soliani, M. Synthesis of 4H-3,1-benzoxazines, quinazolin-2-ones, and quinoline-4-ones by palladium-catalyzed oxidative carbonylation of 2-ethynylaniline derivatives. J. Org. Chem. 2004, 69, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.S.; Babu, R.A.; Reddy, M.R.; Reddy, B.J.M.; Sridhar, B. Intramolecular C–O/C–S bond insertion of α-diazoesters for the synthesis of 2-aryl-4H-benzo[d][1,3]oxazine and 2-aryl-4H-benzo[d][1,3]thiazine derivatives. RSC Adv. 2014, 4, 44629–44633. [Google Scholar] [CrossRef]
- Dias, N.; Goossens, J.-F.; Baldeyrou, B.; Lansiaux, A.; Colson, P.; Di Salvo, A.; Bernal, J.; Turnbull, A.; Mincher, D.J.; Bailly, C. Oxoazabenzo[de]anthracenes conjugated to amino acids: Synthesis and evaluation as DNA-binding antitumor agents. Bioconjugate Chem. 2005, 16, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.J.; Caprathe, B.W.; Gilmore, J.L.; Amin, N.; Emmerling, M.R.; Michael, W.; Nadimpalli, R.; Nath, R.; Raser, K.J.; Stafford, D.; et al. 2-Amino-4H-3,1-benzoxazin-4-ones as Inhibitors of C1r Serine Protease. J. Med. Chem. 1998, 41, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Jarvest, R.L.; Parratt, M.J.; Debouck, C.M.; Gorniak, J.G.; Jennings, L.J.; Serafinowska, H.T.; Strickler, J.E. Inhibition of HSV-1 protease by benzoxazinones. Bioorg. Med. Chem. Lett. 1996, 6, 2463–2466. [Google Scholar] [CrossRef]
- Krantz, A.; Spencer, R.W.; Tam, T.F.; Liak, T.J.; Copp, L.J.; Thomas, E.M.; Rafferty, S.P. Design and synthesis of 4H-3,1-benzoxazin-4-ones as potent alternate substrate inhibitors of human leukocyte elastase. J. Med. Chem. 1990, 33, 464–479. [Google Scholar] [CrossRef] [PubMed]
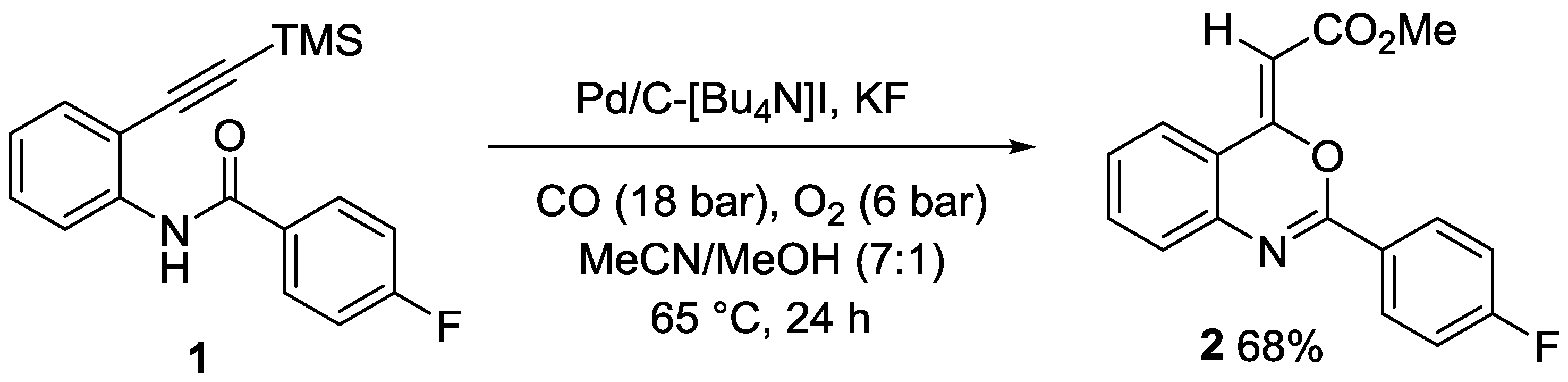
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pancrazzi, F.; Motti, E.; Costa, M.; Mancuso, R.; Gabriele, B.; Della Ca’, N. (Z)-4-(Carbomethoxymethylene)-2-(4-fluorophenyl)-4H-benzo[d][1,3]oxazine. Molbank 2017, 2017, M927. https://doi.org/10.3390/M927
Pancrazzi F, Motti E, Costa M, Mancuso R, Gabriele B, Della Ca’ N. (Z)-4-(Carbomethoxymethylene)-2-(4-fluorophenyl)-4H-benzo[d][1,3]oxazine. Molbank. 2017; 2017(1):M927. https://doi.org/10.3390/M927
Chicago/Turabian StylePancrazzi, Francesco, Elena Motti, Mirco Costa, Raffaella Mancuso, Bartolo Gabriele, and Nicola Della Ca’. 2017. "(Z)-4-(Carbomethoxymethylene)-2-(4-fluorophenyl)-4H-benzo[d][1,3]oxazine" Molbank 2017, no. 1: M927. https://doi.org/10.3390/M927







