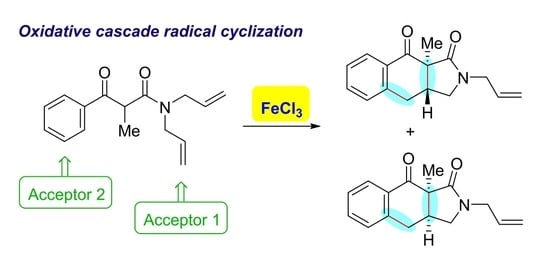
Oxidative Radical Cyclization–Cyclization Reaction Leading to 1H-Benzo[f]isoindole Derivatives
Abstract
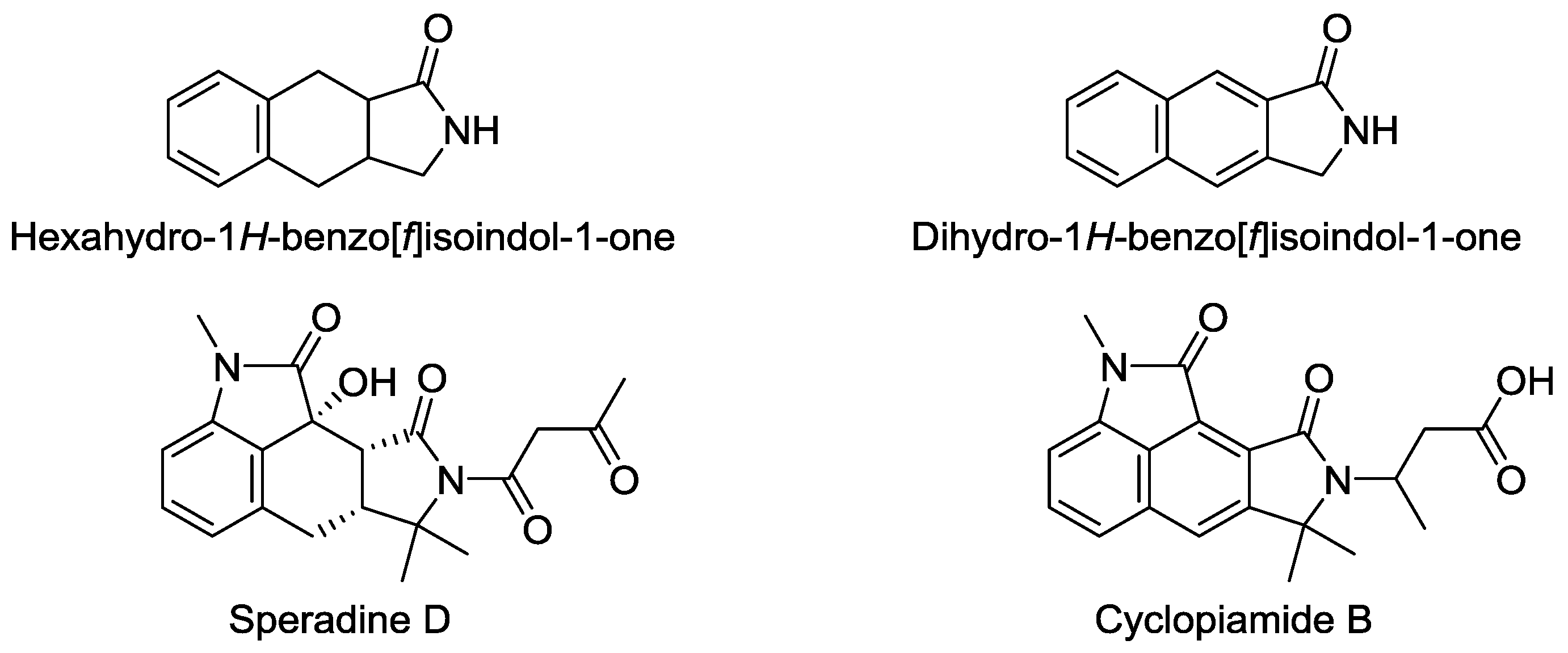
:1. Introduction
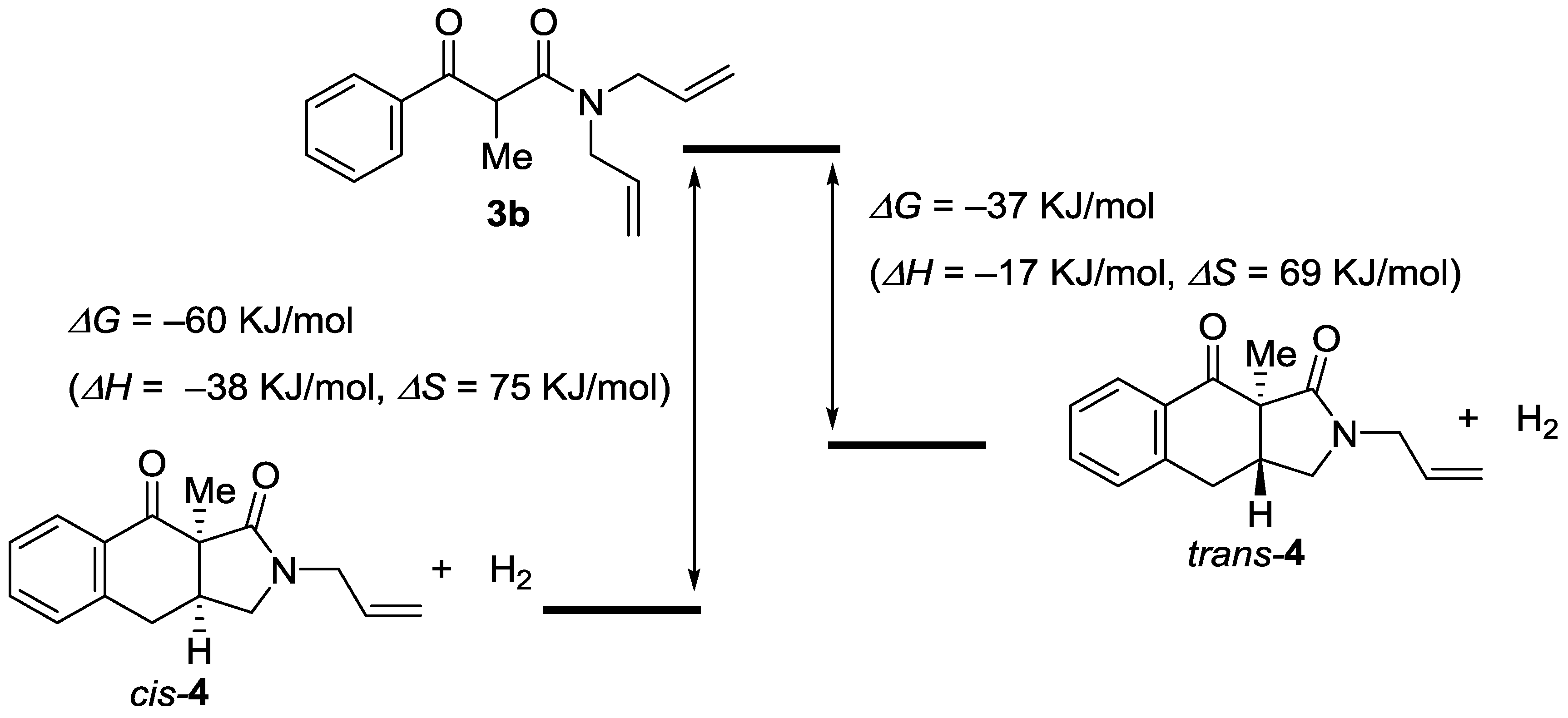
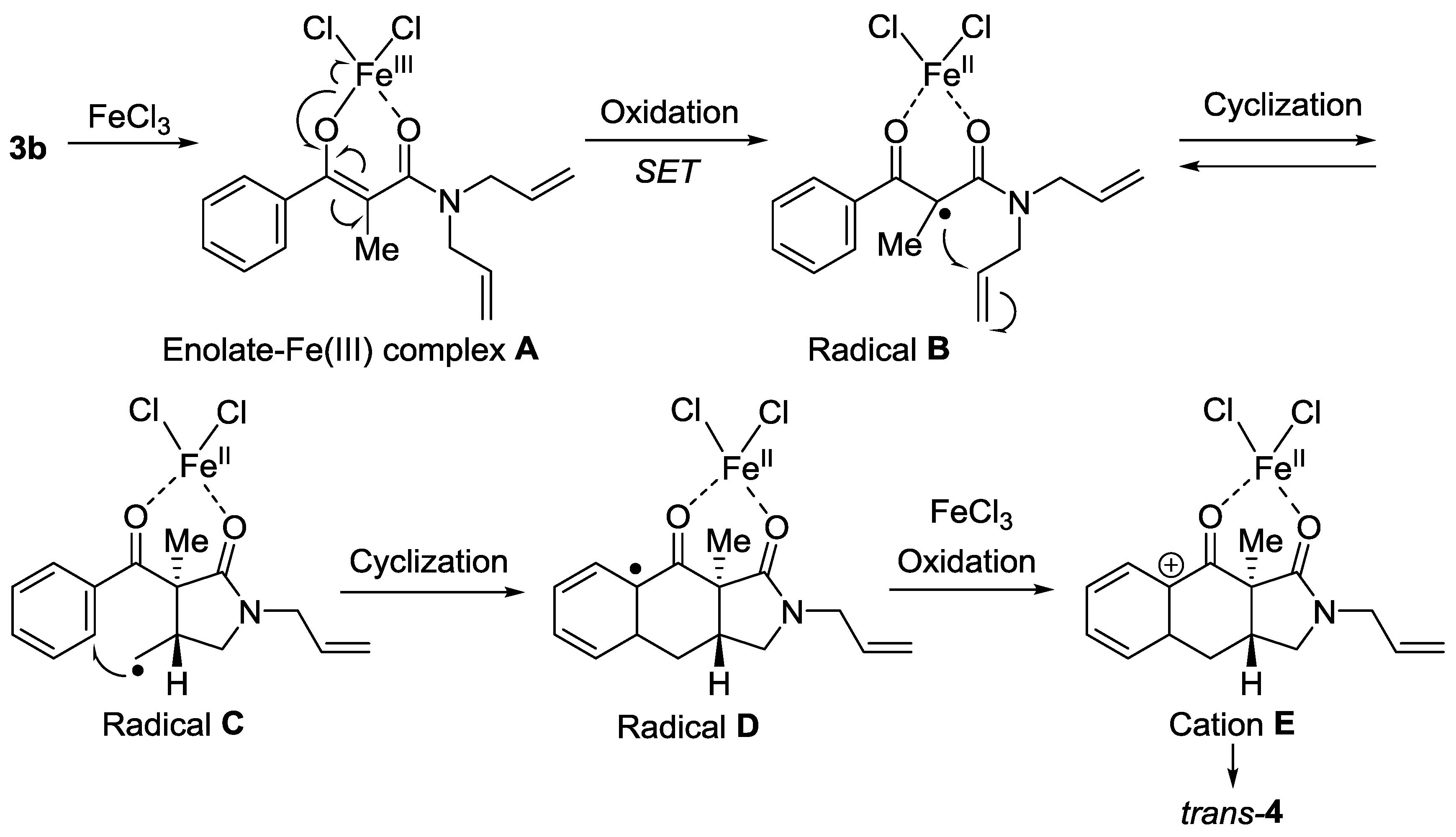
2. Results
3. Discussion
4. Experimental Section
4.1. General Information
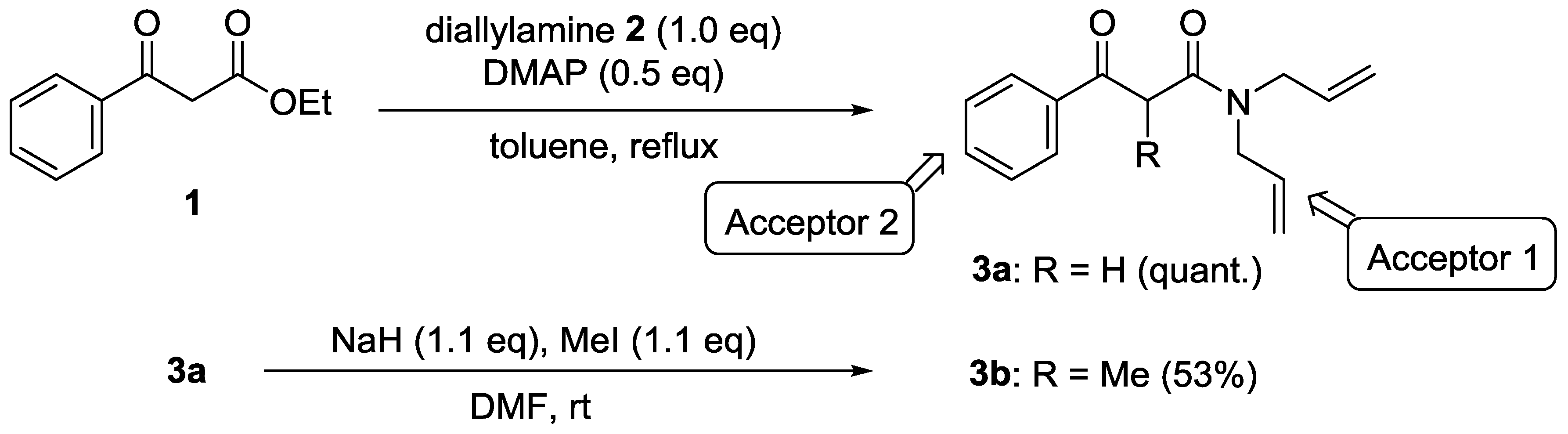
4.2. N,N-Diallyl-3-oxo-3-phenylpropanamide (3a)
4.3. N,N-Diallyl-2-methyl-3-oxo-3-phenylpropanamide (3b)
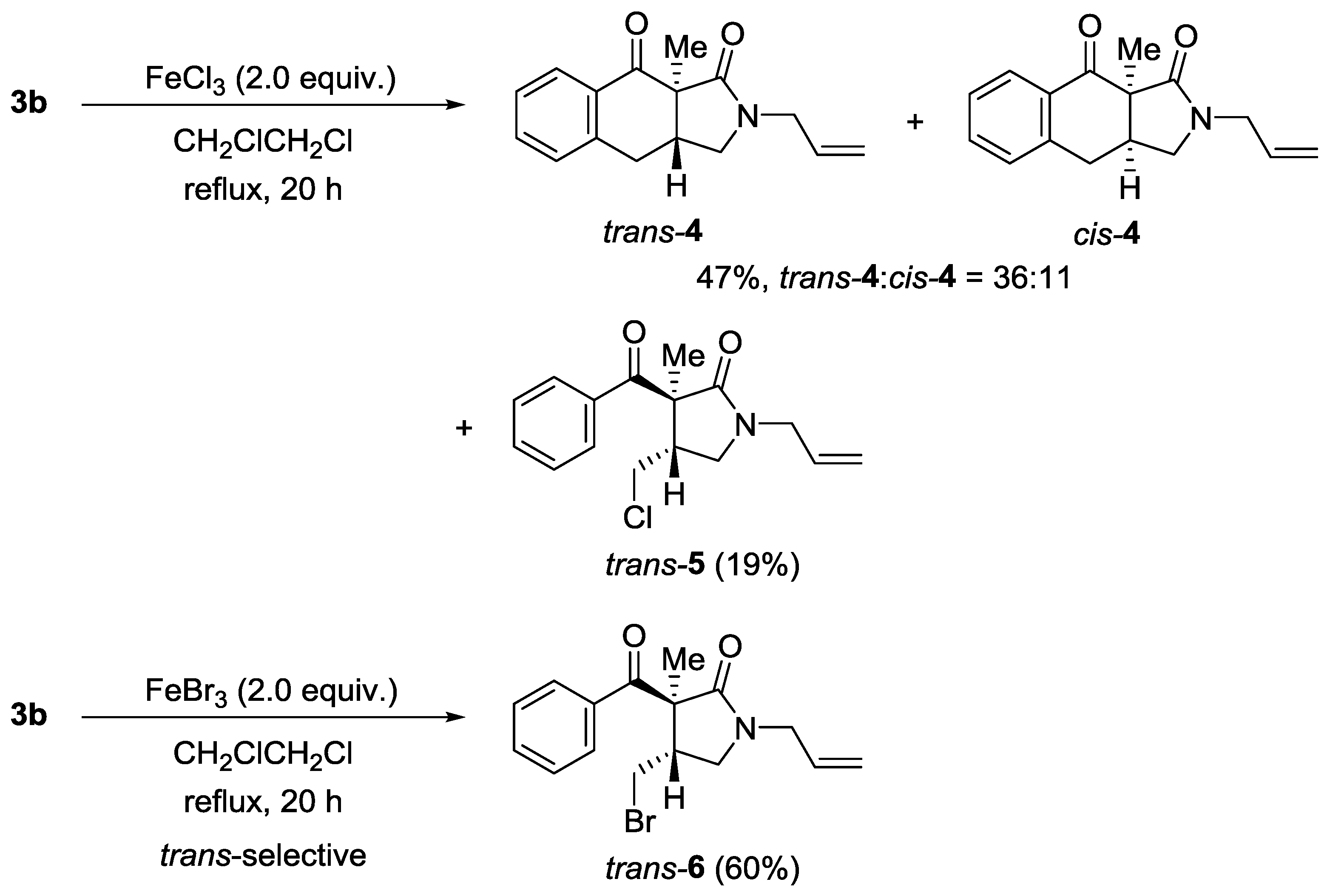
4.4. 2-Allyl-9a-methyl-2,3,3a,9a-tetrahydro-1H-benzo[f]isoindole-1,9(4H)-dione (4) and trans-1-Allyl-3-benzoyl-4-(chloromethyl)-3-methylpyrrolidin-2-one (trans-5)
4.5. trans-1-Allyl-3-benzoyl-4-(bromomethyl)-3-methylpyrrolidin-2-one (trans-6)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Ma, X.; Peng, J.; Wu, G.; Zhu, T.; Li, G.; Gu, Q.; Li, D. Speradines B-D, oxygenated cyclopiazonic acid alkaloids from the sponge-derived fungus Aspergillus flavus MXH-X104. Tetrahedron 2015, 71, 3522–3527. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Nong, X.; Wei, X.; Qi, S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron 2015, 71, 610–615. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Q.-W.; Zhao, Y.-Y.; Zheng, Q.-H.; Liu, Q.-Y.; Chen, L.; Zhang, Q.-Q. Speradines B-E, four novel tetracyclic oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Heterocycles 2014, 89, 1662–1669. [Google Scholar]
- Burch, J.D.; Farand, J.; Colucci, J.; Sturino, C.; Ducharme, Y.; Friesen, R.W.; Lévesque, J.-F.; Gagné, S.; Wrona, M.; Therien, A.G.; et al. Naphthalene/quinoline amides and sulfonylureas as potent and selective antagonists of the EP4 receptor. Bioorg. Med. Chem. Lett. 2011, 21, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hui, J.; Wang, D.; Zhu, L.; Fang, J.-H.; Zhao, X.-D. Synthesis, cytotoxicity and pro-apoptosis of novel benzoisoindolin hydrazones as anticancer agent. Chem. Pharm. Bull. 2010, 58, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Nishida, Y.; Masuoka, C.; Li, J.-C.; Okawa, M.; Ikeda, T.; Nohara, T. Lignan derivatives and a norditerpene from the seeds of Vitex negundo. J. Nat. Prod. 2004, 67, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Imoto, Y.; Yoshikawa, T.; Kohtani, S.; Miyabe, H. FeCl3–promoted oxidative radical cyclization for the synthesis of lactams having a quaternary carbon. Synlett 2017, 28. in press. [Google Scholar] [CrossRef]
- Snider, B.B. Manganese(III)-based oxidative free-radical cyclizations. Chem. Rev. 1996, 96, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Deepthi, A. Recent advances in CAN mediated reactions in organic synthesis. Tetrahedron 2009, 65, 10745–10755. [Google Scholar] [CrossRef]
- Mohan, R.M.; Kates, S.A.; Dombroski, M.A.; Snider, B.B. Manganese (III) based oxidative free-radical cyclizations. 3. Polycyclization reactions proceeding through secondary radicals. Tetrahedron Lett. 1987, 28, 845–848. [Google Scholar] [CrossRef]
- Curran, D.P.; Morgan, T.M.; Schwartz, C.E.; Snider, B.B.; Dombroski, M.A. Cyclizations of unsaturated ·CR(COX)2 radicals. Manganese(III) acetate oxidative cyclizations of unsaturated acetoacetates and atom-transfer cyclizations of unsaturated haloacetoacetates give the same radicals. J. Am. Chem. Soc. 1991, 113, 6607–6617. [Google Scholar] [CrossRef]
- González, M.A.; Molina-Navarro, S. Attempted synthesis of spongidines by a radical cascade terminating onto a pyridine ring. J. Org. Chem. 2007, 72, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Suda, N.; Hayashi, Y.; Hirama, M. Stereoselective synthesis of zoanthenol ABC-ring by radical strategy. Tetrahedron Lett. 2013, 54, 1389–1391. [Google Scholar] [CrossRef]
- Wong, Y.-C.; Kao, T.-T.; Huang, J.-K.; Jhang, Y.-W.; Chou, M.-C.; Shia, K.-S. Manganese(III)-catalyzed oxidative cyclization of aryl 1-cyanoalk-5-ynyl ketone systems: A convenient and general approach to cyclopenta[b]naphthalene derivatives. Adv. Synth. Catal. 2014, 356, 3025–3038. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Tan, X.; Lu, J.; Cormier, K.W.; Ma, Z.; Chen, C. A biomimetic route for construction of the [4+2] and [3+2] core skeletons of dimeric pyrrole-imidazole alkaloids and asymmetric synthesis of ageliferins. J. Am. Chem. Soc. 2012, 134, 18834–18842. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.O.; Bolm, C. Iron(III) chloride in oxidative C–C coupling reactions. Chem. Soc. Rev. 2009, 38, 2730–2744. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-R.; Cheng, L.; Kong, D.-L.; Huang, H.-Y.; Gu, C.-L.; Liu, L.; Wang, D.; Li, C.-J. FeCl3‑mediated radical tandem reactions of 3‑benzyl-2-oxindoles with styrene derivatives for the stereoselective synthesis of spirocyclohexene oxindoles. Org. Lett. 2016, 18, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ishibashi, H. Iron-mediated radical nitro-cyclization reaction of 1,6-dienes. Org. Lett. 2010, 12, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.F.; Velu, S.S.; Weber, J.-F.F.; Lee, K.C.; Hadi, A.H.A.; Richomme, P.; Rondeau, D.; Noorbatcha, I.; Awang, K. A tandem highly stereoselective FeCl3-promoted synthesis of a bisindoline: synthetic utility of radical cations in heterocyclic construction. Tetrahedron 2004, 60, 11733–11742. [Google Scholar] [CrossRef]
- Booker-Milburn, K.I.; Barker, A.; Brailsford, W.; Cox, B.; Mansley, T.E. Fe(III) mediated oxidative radical cyclisation of cyclopropanone acetals. Tetrahedron 1998, 54, 15321–15344. [Google Scholar] [CrossRef]
- Cossy, J.; Belotti, D.; Cuong, N.K.; Chassagnard, C. Photoreductive cyclization of N,N-dialkyl-β-oxoamides: synthesis of piperidines and δ-lactams. Tetrahedron 1993, 49, 7691–7700. [Google Scholar] [CrossRef]
- See: Supplementary Material.
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, E.; Miyabe, H. Oxidative Radical Cyclization–Cyclization Reaction Leading to 1H-Benzo[f]isoindole Derivatives. Molbank 2017, 2017, M929. https://doi.org/10.3390/M929
Yoshioka E, Miyabe H. Oxidative Radical Cyclization–Cyclization Reaction Leading to 1H-Benzo[f]isoindole Derivatives. Molbank. 2017; 2017(1):M929. https://doi.org/10.3390/M929
Chicago/Turabian StyleYoshioka, Eito, and Hideto Miyabe. 2017. "Oxidative Radical Cyclization–Cyclization Reaction Leading to 1H-Benzo[f]isoindole Derivatives" Molbank 2017, no. 1: M929. https://doi.org/10.3390/M929