7-Bromo-1-methyl-2-phenyl-1H-indole-3-carbonitrile
Abstract
:1. Introduction
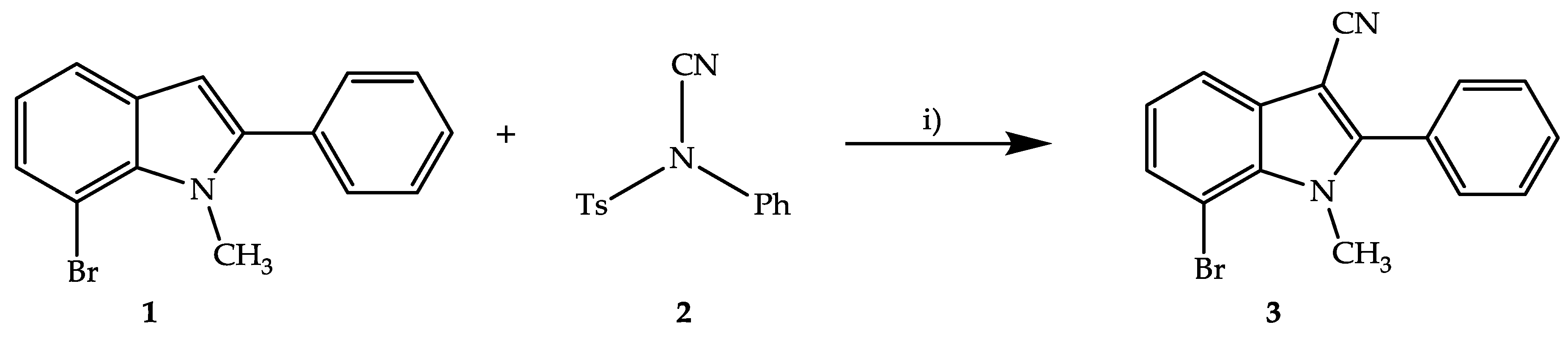
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Anal. | elemental analysis |
| APCI-MS | atmospheric pressure chemical ionisation mass spectrometry |
| HPLC | high performance liquid chromatography |
| IR | infrared spectrometry |
| NMR | nuclear magnetic resonance |
| TLC | thin layer chromatography |
| TMS | tetramethylsilane |
References
- Roskoski, R., Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kunick, C.; Egert-Schmidt, A.-M. Young, competitive, successful: A short history of protein kinase inhibitors. Pharm. Unserer Zeit 2008, 37, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Tolle, N.; Kunick, C. Paullones as inhibitors of protein kinases. Curr. Top. Med. Chem. 2011, 11, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Falke, H.; Bumiller, K.; Harbig, S.; Masch, A.; Wobbe, J.; Kunick, C. 2-tert-Butyl-5,6,7,8,9,10-hexahydrocyclohepta[b]indole. Molbank 2011, 2011, M737. [Google Scholar] [CrossRef]
- Schmidt, S.; Preu, L.; Lemcke, T.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Kunick, C. Dual IGF-1R/SRC inhibitors based on a N′-aroyl-2-(1H-indol-3-yl)-2-oxoacetohydrazide structure. Eur. J. Med. Chem. 2011, 46, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Meine, R.; Falke, H.; Kötz, J.; Schweda, S.I.; Kunick, C. 7-Iodo-1H-indole-3-carbonitrile. Molbank 2015, 2015, M869. [Google Scholar] [CrossRef]
- McGrath, C.F.; Pattabiraman, N.; Kellogg, G.E.; Lemcke, T.; Kunick, C.; Sausville, E.A.; Zaharevitz, D.W.; Gussio, R. Homology model of the CDK1/cyclin B complex. J. Biomol. Struct. Dyn. 2005, 22, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Tellers, D.M.; Streckfuss, E.C.; Chen, C.-Y.; Davies, I.W. [1,2]-Aryl migration in the synthesis of substituted indoles. Scope, mechanism, and high throughput experimentation. Tetrahedron 2009, 65, 3285–3291. [Google Scholar] [CrossRef]
- Islam, S.; Larrosa, I. “On water”, phosphine-free palladium-catalyzed room temperature C-H arylation of indoles. Chem. Eur. J. 2013, 19, 15093–15096. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Dhar, D.N.; Suri, S.C. Reaction of indoles with chlorosulphonyl isocyanate; a versatile route to 3-substituted indoles. Synthesis 1978, 1978, 374–376. [Google Scholar] [CrossRef]
- Kurzer, F. Cyanamides. Part I. The synthesis of substituted arylsulphonylcyanamides. J. Chem. Soc. 1949, 1034–1038. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, J. Lewis acid catalyzed direct cyanation of indoles and pyrroles with N-cyano-N-phenyl-p-toluenesulfonamide (NCTS). Org. Lett. 2011, 13, 5608–5611. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meine, R.; Blech, M.; Lindhof, J.; Kunick, C. 7-Bromo-1-methyl-2-phenyl-1H-indole-3-carbonitrile. Molbank 2017, 2017, M941. https://doi.org/10.3390/M941
Meine R, Blech M, Lindhof J, Kunick C. 7-Bromo-1-methyl-2-phenyl-1H-indole-3-carbonitrile. Molbank. 2017; 2017(2):M941. https://doi.org/10.3390/M941
Chicago/Turabian StyleMeine, Rosanna, Max Blech, Jens Lindhof, and Conrad Kunick. 2017. "7-Bromo-1-methyl-2-phenyl-1H-indole-3-carbonitrile" Molbank 2017, no. 2: M941. https://doi.org/10.3390/M941




