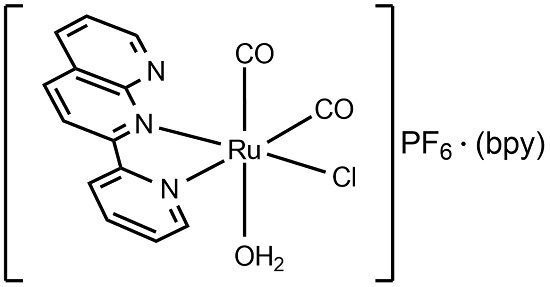
(OC-6-35-A)-Aquadicarbonylchlorido[2-(2-pyridyl)-1,8-naphthyridine-κ;2N1,N2]ruthenium(II) hexafluoridophosphate 2,2′-Bipyridine
Abstract
:1. Introduction
2. Results and Discussion
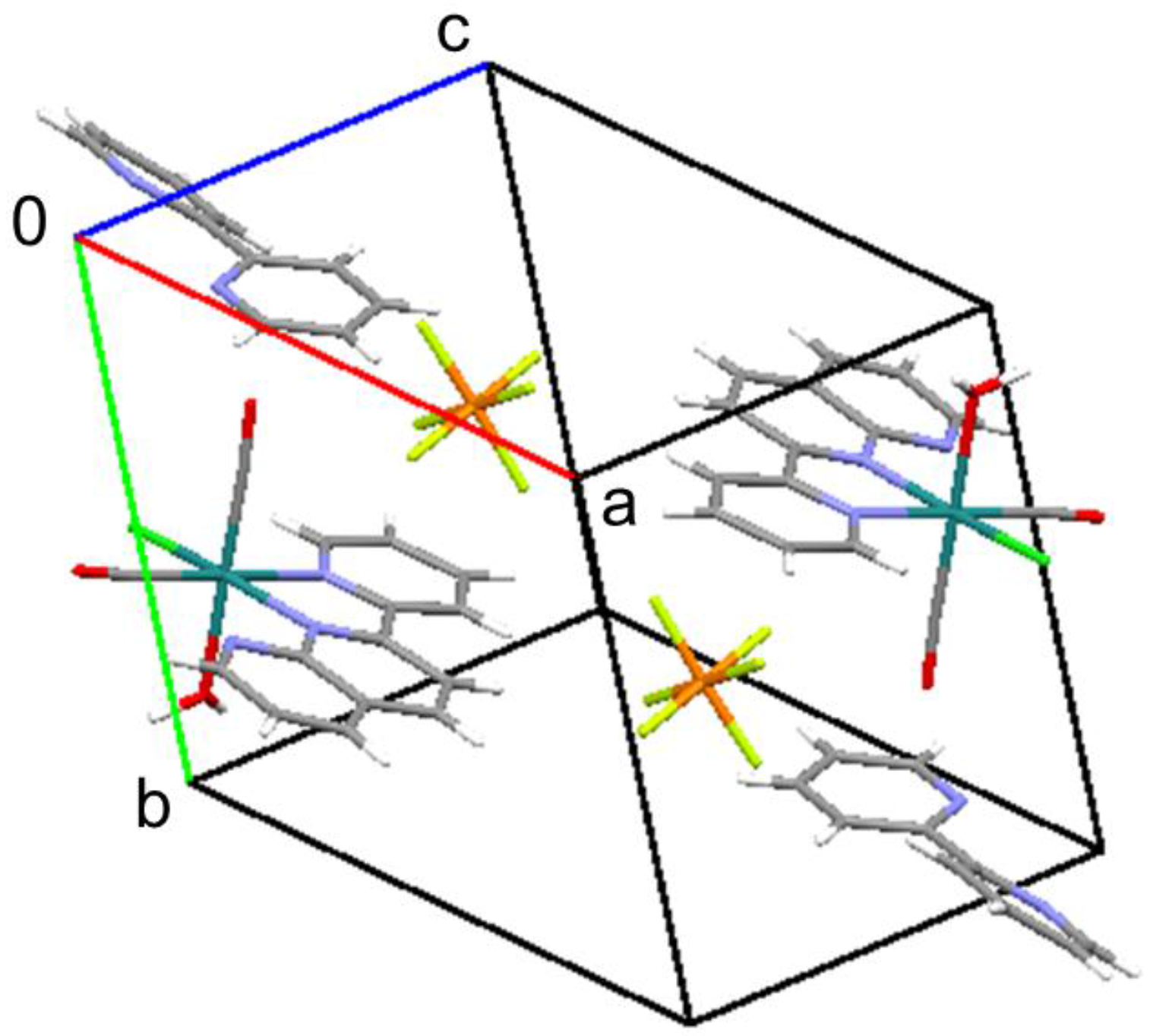
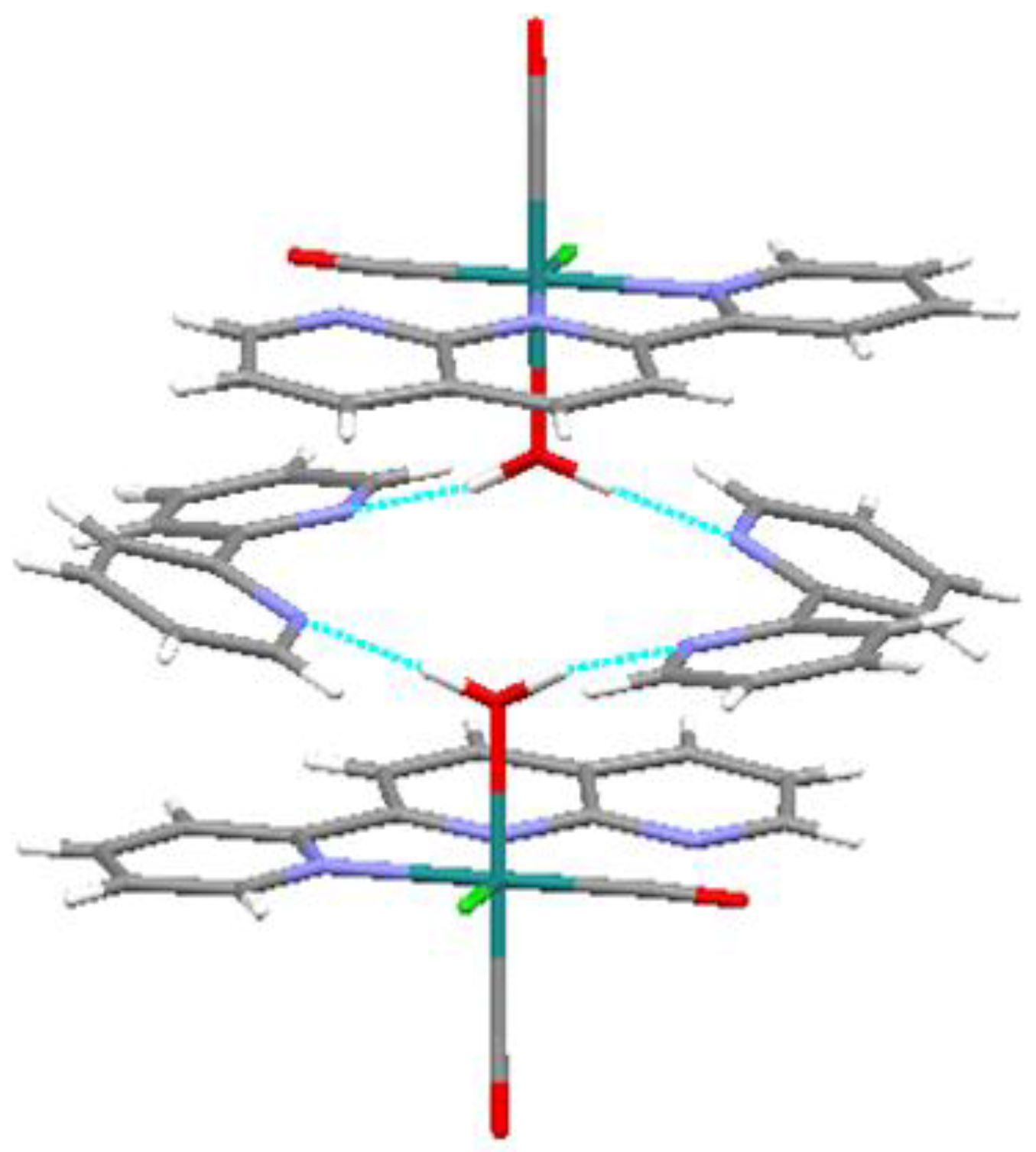
2.1. Isolation and Structure of the Title Compound
2.2. Experimental and Calculated IR Spectra
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of [Ru(pynp)(CO)2(OH2)Cl]PF6
3.3. Computational Details
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Mitsudo, T.; Suzuki, N.; Kondo, T.; Watanabe, Y. Ru3(CO)12/1,10-phenanthroline-catalyzed hydroformylation of α-olefins. J. Mol. Catal. A. 1996, 109, 219–225. [Google Scholar] [CrossRef]
- Moya, S.A.; Gajardo, J.; Araya, J.C.; Cornejo, J.J.; Guerchais, V.; Le Bozec, H.; Bayón, J.C.; Pardey, A.J.; Aguirre, P. Synthesis and characterization of new complexes of the type [Ru(CO)2Cl2(2-phenyl-1,8-naphthyridine-κ;N)(2-phenyl-1,8-naphthyridine-κ;N’)]. Preliminary applications in homogeneous catalysis. Appl. Organometal. Chem. 2008, 22, 471–478. [Google Scholar] [CrossRef]
- Oyama, D.; Hamada, T.; Takase, T. Stereospecific synthesis and redox properties of ruthenium(II) carbonyl complexes bearing a redox-active 1,8-naphthyridine unit. J. Organomet. Chem. 2011, 696, 2263–2268. [Google Scholar] [CrossRef]
- Oyama, D.; Yuzuriya, K.; Naoi, R.; Hamada, T.; Takase, T. Syntheses of geometrical isomers for comparison of properties caused by steric and electronic effects in carbonylruthenium(II) complexes. Bull. Chem. Soc. Jpn. 2014, 87, 1107–1115. [Google Scholar] [CrossRef]
- Oyama, D.; Abe, R.; Takase, T. CO-ligand photodissociation in two Ru(II) complexes affected by different polypyridyl supporting ligands. Chem. Lett. (in press). [CrossRef]
- Anderson, P.A.; Deacon, G.B.; Haarmann, K.H.; Keene, F.R.; Meyer, T.J.; Reitsma, D.A.; Skelton, B.W.; Strouse, G.F.; Thomas, N.C.; Treadway, J.A.; et al. Designed synthesis of mononuclear tris(heteroleptic) ruthenium complexes containing bidentate polypyridyl ligands. Inorg. Chem. 1995, 34, 6145–6157. [Google Scholar] [CrossRef]
- Oyama, D.; Hamada, T. cis,trans-Dicarbonyldichlorido[2-(2-pyridyl)-1,8-naphthyridine-κ;N1,N2]ruthenium(II). Acta Cryst. 2008, E64, m442–m443. [Google Scholar] [CrossRef] [PubMed]
- Oyama, D.; Yamanaka, T.; Fukuda, A.; Takase, T. Modulation of intramolecular hydrogen-bonding strength by axial ligands in ruthenium(II) complexes. Chem. Lett. 2013, 42, 1554–1555. [Google Scholar] [CrossRef]
- Oyama, D.; Asuma, A.; Hamada, T.; Takase, T. Novel [Ru(polypyridine)(CO)2Cl2] and [Ru(polypyridine)2(CO)Cl]+-type complexes: Characterizing the effects of introducing azopyridyl ligands by electrochemical, spectroscopic and crystallographic measurements. Inorg. Chim. Acta 2009, 362, 2581–2588. [Google Scholar] [CrossRef]
- Oyama, D.; Suzuki, K.; Yamanaka, T.; Takase, T. One-pot synthesis of cis-bis(2,2′-bipyridine) carbonylruthenium(II) complexes from a carbonato precursor: X-ray crystal structures and electron transfer processes of the series complexes. J. Coord. Chem. 2012, 65, 78–86. [Google Scholar] [CrossRef]
- Takase, T.; Takahashi, K.; Oyama, D. cis,trans-Dicarbonyldichlorido(1,10-phenanthroline-5,6-dione-κ;2N,N’)ruthenium(II). IUCrData 2017, 2, x170288. [Google Scholar] [CrossRef]
- Oyama, D.; Ukawa, N.; Hamada, T.; Takase, T. Reversible intramolecular cyclization in ruthenium complexes induced by ligand-centered one-electron transfer on bidentate naphthyridine: An important intermediate for both metal- and organo-hydride species. Chem. Lett. 2015, 44, 533–535. [Google Scholar] [CrossRef]
- Abe, R.; Takase, T.; Oyama, D. 2-(Pyridin-2-yl) pyridinium trifluoromethanesulfonate. IUCrData 2017, 2, x170689. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley & Sons: New York, USA, 1986; pp. 291–308. [Google Scholar]
- Rigaku. CrystalStructure; Version 4.0; Rigaku Corporation: Tokyo, Japan, 2010. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Campos-Fernández, C.S.; Thomson, L.M.; Galán-Mascarós, J.R.; Ouyang, X.; Dunbar, K.R. Homologous series of redox-active, dinuclear cations [M2(O2CCH3)2(pynp)2]2+ (M = Mo, Ru, Rh) with the bridging ligand 2-(2-pyridyl)-1,8-naphthyridine (pynp). Inorg. Chem. 2002, 41, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]

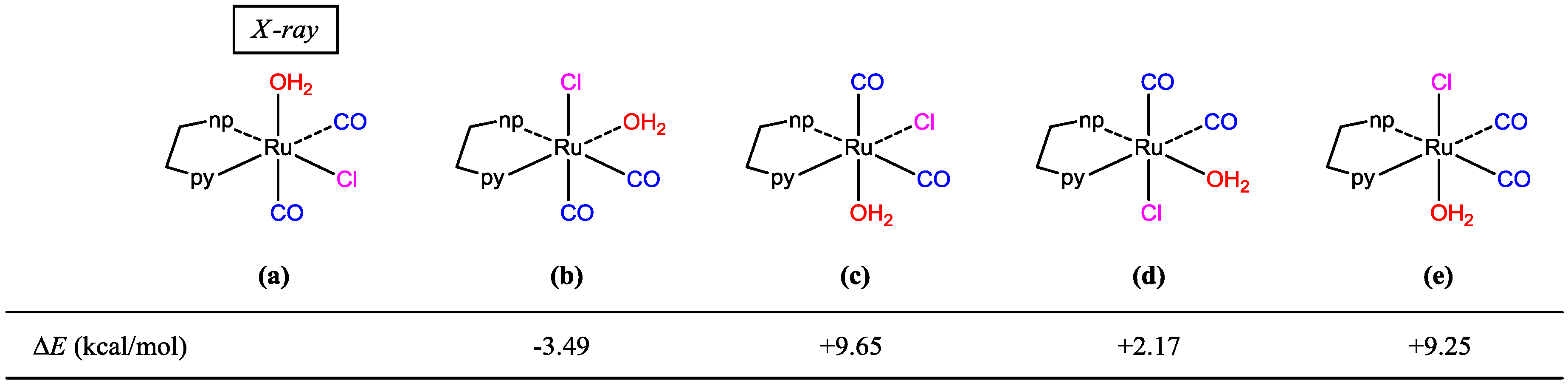

| Parameters | Expt. | (a) | (b) | (c) | (d) | (e) |
|---|---|---|---|---|---|---|
| Bond lengths | ||||||
| Ru–O | 2.114(3) | 2.238 | 2.169 | 2.243 | 2.207 | 2.230 |
| Ru–Cl | 2.4002(12) | 2.428 | 2.433 | 2.438 | 2.446 | 2.411 |
| Ru–C1 | 1.860(5) | 1.883 | 1.922 | 1.888 | 1.918 | 1.913 |
| Ru–C2 | 1.910(5) | 1.939 | 1.926 | 1.923 | 1.944 | 1.926 |
| Ru–Npy 1 | 2.128(4) | 2.146 | 2.089 | 2.121 | 2.127 | 2.164 |
| Ru–Nnp 2 | 2.074(4) | 2.139 | 2.173 | 2.172 | 2.095 | 2.160 |
| C1–O1 | 1.134(6) | 1.146 | 1.146 | 1.144 | 1.147 | 1.146 |
| C2–O2 | 1.073(5) | 1.143 | 1.143 | 1.144 | 1.143 | 1.144 |
| Bond angles | ||||||
| Ru–C1–O1 | 176.0(4) | 178.7 | 179.3 | 179.9 | 179.7 | 177.4 |
| Ru–C2–O2 | 171.6(5) | 174.3 | 178.5 | 178.5 | 172.2 | 176.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takase, T.; Abe, R.; Oyama, D. (OC-6-35-A)-Aquadicarbonylchlorido[2-(2-pyridyl)-1,8-naphthyridine-κ;2N1,N2]ruthenium(II) hexafluoridophosphate 2,2′-Bipyridine. Molbank 2017, 2017, M950. https://doi.org/10.3390/M950
Takase T, Abe R, Oyama D. (OC-6-35-A)-Aquadicarbonylchlorido[2-(2-pyridyl)-1,8-naphthyridine-κ;2N1,N2]ruthenium(II) hexafluoridophosphate 2,2′-Bipyridine. Molbank. 2017; 2017(3):M950. https://doi.org/10.3390/M950
Chicago/Turabian StyleTakase, Tsugiko, Ryosuke Abe, and Dai Oyama. 2017. "(OC-6-35-A)-Aquadicarbonylchlorido[2-(2-pyridyl)-1,8-naphthyridine-κ;2N1,N2]ruthenium(II) hexafluoridophosphate 2,2′-Bipyridine" Molbank 2017, no. 3: M950. https://doi.org/10.3390/M950