(E)-2-(1-Cyano-2-methoxy-2-oxoethylidene)-3,4-dioxo-1-(pyridin-1-ium-1-yl)cyclobutan-1-ide
Abstract
:1. Introduction
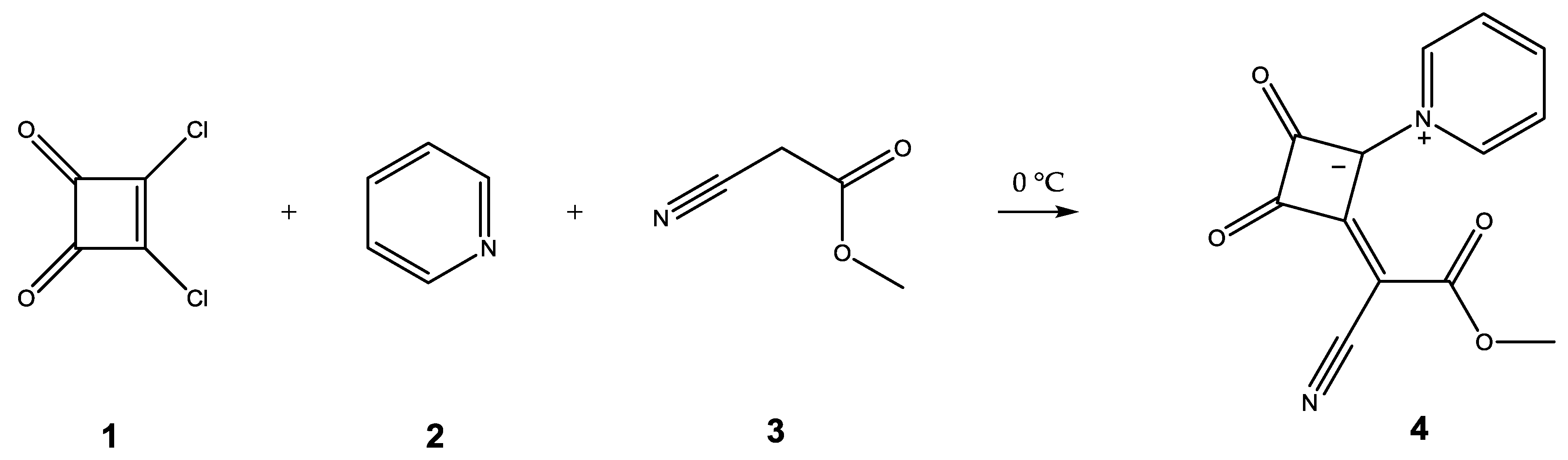
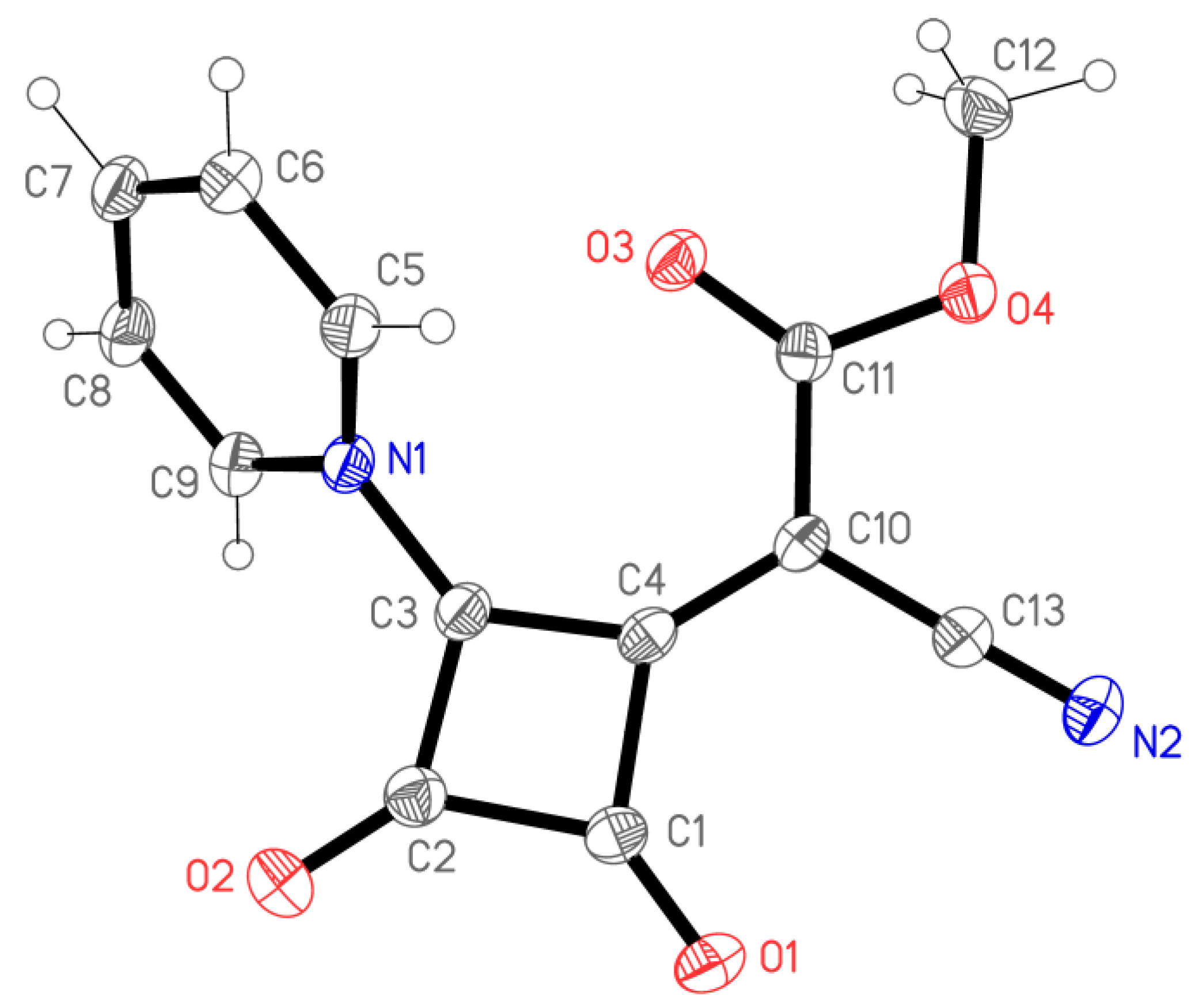
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis
3.4. X-ray Structure Determination of 4
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- West, R.; Powell, D.L. New aromatic anions. III. Molecular orbital calculations on oxygenated anions. J. Am. Chem. Soc. 1963, 85, 2577–2579. [Google Scholar] [CrossRef]
- Fatiadi, A.J. Synthesis of 1,3-(dicyanomethylene)croconate salts. New bond-delocalized dianion, “Croconate Violet”. J. Am. Chem. Soc. 1978, 100, 2586–2587. [Google Scholar] [CrossRef]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Shafeekh, K.M.; Das, S.; Sissa, C.; Painelli, A. Asymmetric squaraine dyes: Spectroscopic and theoretical investigation. J. Phys. Chem. B 2013, 117, 8536–8546. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-Y.; Su, C.; Akula, S.B.; Sun, W.-G.; Chien, H.-M.; Li, W.-R. New pyridinium ylide dyes for dye sensitized solar cell applications. Org. Lett. 2016, 18, 3386–3389. [Google Scholar]
- Kolev, T.M.; Yancheva, D.Y.; Stoyanov, S.I. Synthesis and spectral and structural elucidation of some pyridinium betaines of squaric acid: Potential materials for nonlinear optical applications. Adv. Funct. Mater. 2004, 14, 799–805. [Google Scholar] [CrossRef]
- Grünefeld, J.; Zinner, G. Neue Oxokohlenstoff-Betaine der Quadratsäure. Chem.-Ztg. 1984, 108, 112. [Google Scholar]
- Schmidt, A.H.; Aimène, A.; Schneider, M. Eine neue Klasse von Stickstoff-Betainen der Quadratsäure. Synthesis 1984, 436–439. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Straus, M.; Botzet, D. Oxokohlenstoffe und verwandte Verbindungen; 10. Mitteilung. Pseudooxokohlenstoff-Betaine der C4-Reihe mit einer Cyanamino- bzw. einer Sulfonylamino-Funktion durch Dreikomponenten-Reaktion. Synthesis 1985, 1055–1057. [Google Scholar] [CrossRef]
- Lunelli, B. New, optimized preparation of 1,2-dichlorocyclobuten-1,2-dione (C4O2Cl2) from squaric acid and oxalyl chloride. Tetrahedron Lett. 2007, 48, 3595–3597. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünefeld, J.; Kunick, C.; Jones, P.G. (E)-2-(1-Cyano-2-methoxy-2-oxoethylidene)-3,4-dioxo-1-(pyridin-1-ium-1-yl)cyclobutan-1-ide. Molbank 2017, 2017, M953. https://doi.org/10.3390/M953
Grünefeld J, Kunick C, Jones PG. (E)-2-(1-Cyano-2-methoxy-2-oxoethylidene)-3,4-dioxo-1-(pyridin-1-ium-1-yl)cyclobutan-1-ide. Molbank. 2017; 2017(3):M953. https://doi.org/10.3390/M953
Chicago/Turabian StyleGrünefeld, Johann, Conrad Kunick, and Peter G. Jones. 2017. "(E)-2-(1-Cyano-2-methoxy-2-oxoethylidene)-3,4-dioxo-1-(pyridin-1-ium-1-yl)cyclobutan-1-ide" Molbank 2017, no. 3: M953. https://doi.org/10.3390/M953




