(E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one
Abstract
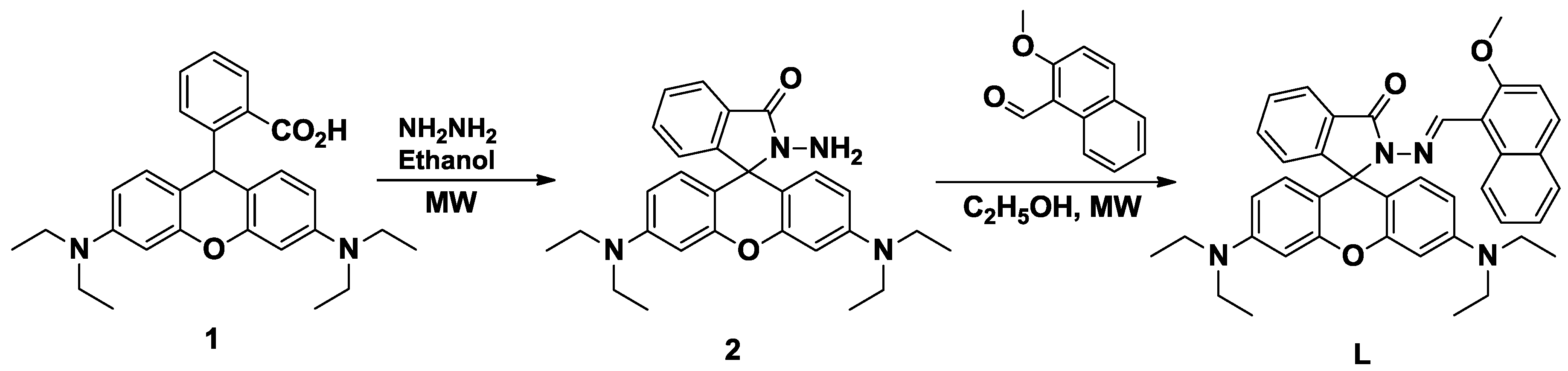
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 2-Amino-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (2)
3.3. Synthesis of (E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one (L)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Lohar, S.; Banerjee, A.; Sahana, A.; Banik, A.; Mukhopadhyay, S.; Das, D. A rhodamine-naphthalene conjugate as a FRET based sensor for Cr3+ and Fe3+ with cell staining application. Anal. Methods 2013, 5, 442–445. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sasaoka, N.; Tsuchida, T.; Fujiware, T.; Nagao, S.; Ohmoto, T. Fluorescent dye rhodamine 6G as a molecular probe to study drug resistance of C6 rat glioma cells. J. Neuro-Oncol. 1992, 13, 217–222. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.; Franz, K. A cell-permeable fluorescent prochelator responds to hydrogen peroxide and metal ions by decreasing fluorescence. Inorg. Chim. Acta 2012, 380, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Aydin, Z.; Zhang, Y.; Liu, Z.; Guo, M. A turn-on fluorescent sensor for imaging labile Fe3+ in live neuronal cells at subcellular resolution. ChemBioChem 2012, 13, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, P.; Yan, W.; He, C.; Xiong, L.; Li, F.; duan, C. A bright water-compatible sugar-rhodamine fluorescence sensor for selective detection oh Hg2+ in natural water and living cells. J. Environ. Monit. 2009, 11, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hue, F.J.; Su, J.; Sun, Y.Q.; Yin, C.X.; Tong, H.B.; Nie, Z.X. A rhodamine-based dual chemosensor for the visual detection of copper and the ratiometric fluorescent detection of vanadium. Dyes Pigment. 2010, 86, 50–55. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Qian, X. A rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: A NS2-containing receptor. J. Org. Chem. 2009, 74, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, A.J.; Abebe, F.A.; Venter, A.; Sinn, E. Rhodamine based turn-on sensors for Cr3+ and Ni2+: Detecting CN− via the metal displacement approach of sensor-Cr3+ complex. J. Fluoresc. 2016, 26, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, A.; Abebe, F.; Sinn, E. Rhodamine based turn-on dual sensor for Fe3+ and Cu2+. Tetrahedron Lett. 2011, 52, 5648–5651. [Google Scholar] [CrossRef]
- Wang, H.H.; Xue, L.; Yu, C.L.; Qian, Y.Y.; Jiang, H. Rhodamine-based fluorescent sensor for mercury in buffer solution and living cells. Dyes Pigment. 2011, 91, 350–355. [Google Scholar] [CrossRef]
- Abebe, F.; Sinn, E. Fluorescein-based fluorescent and colorimetric chemosensors for copper in aqueous media. Tetrahedron Lett. 2011, 52, 5234–5237. [Google Scholar] [CrossRef]
- Abebe, F.; Eribal, C.; Ramakrishna, G.; Sinn, E.A. “Turn-on” fluorescent sensor for the selective detection of Cobalt and Nickel ions in aqueous media. Tetrahedron Lett. 2011, 52, 5554–5558. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanism, and applications in living cells. J. Am. Chem. Soc. 2009, 132, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.; Silva, A.; Cunha-Silvs, L.; Castro, B.; Gameiro, P.; Rangel, M. Discrimination of fluorescence light-up effects induced by PH and metal ion chelation on a spirocyclic derivative of rhodamine B. Dalton Trans. 2013, 42, 6110–6118. [Google Scholar] [CrossRef] [PubMed]
| Trial | Method | Temp. (°C) | Pressure (Bar) | Hold Time (min) | Yield a (%) |
|---|---|---|---|---|---|
| 1 | MW in ethanol | 100 | 8.3 | 10 | 92 |
| 2 | MW in ethanol | 80 | 6.7 | 10 | 86 |
| 3 | MW in ethanol | 80 | 6.7 | 20 | 74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins, P.; Winstead, A.; Abebe, F. (E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one. Molbank 2017, 2017, M955. https://doi.org/10.3390/M955
Perkins P, Winstead A, Abebe F. (E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one. Molbank. 2017; 2017(3):M955. https://doi.org/10.3390/M955
Chicago/Turabian StylePerkins, Pierce, Angela Winstead, and Fasil Abebe. 2017. "(E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one" Molbank 2017, no. 3: M955. https://doi.org/10.3390/M955




