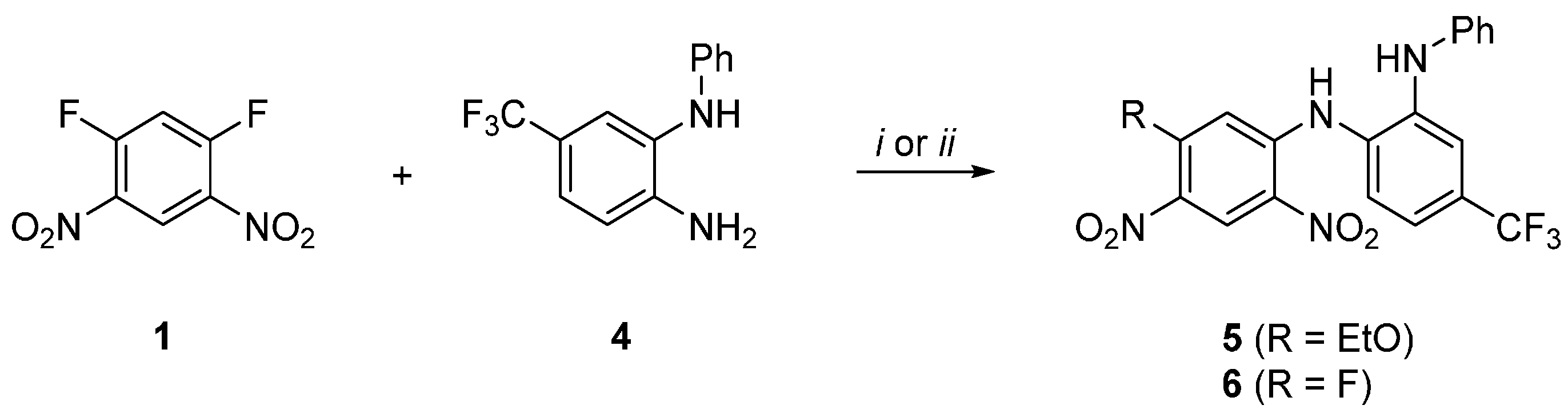
N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine
Abstract
:1. Introduction
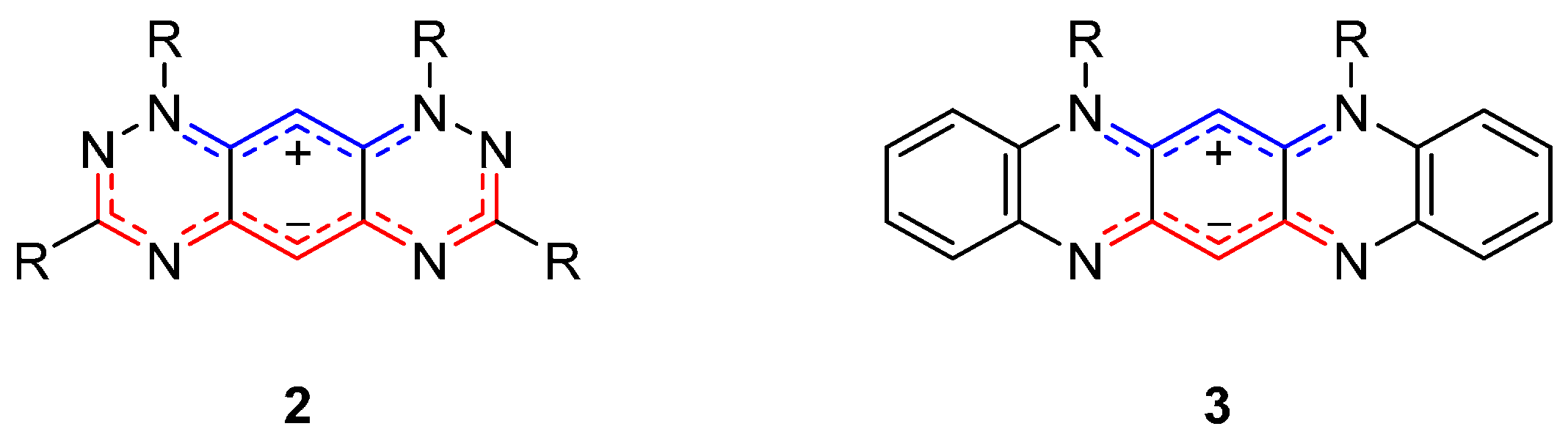
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Method A: N1-(5-Ethoxy-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine (5) and N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine (6)
3.3. Method B: N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine (6)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siele, V.I.; Warman, M. Preparation of 1,3-Difluoro-2,4,6-trinitrobenzene. J. Org. Chem. 1962, 27, 1910–1911. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Zhang, C.; Zhang, H.; Wang, B.; Xu, J.; Fu, L.; Yin, D.; Cooper, C.B.; Ma, Z.; et al. Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents. Molecules 2014, 19, 4380–4394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Liu, G. Solution-Phase Parallel Synthesis of Diverse 1,5-Benzodiazepin-2-ones. J. Comb. Chem. 2007, 9, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, G.; Li, L.; Wang, Z.; Wang, L. Synthesis of Diverse Benzo[1,4]oxazin-3-one-Based Compounds Using 1,5-Difluoro-2,4-dinitrobenzene. J. Comb. Chem. 2007, 9, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, Y.; Chen, Y.; Sun, G.; Wu, X.; Zhang, S.; Han, C.; Wang, G.; Li, L.; Liu, G. Parallel Solution-Phase Synthesis of 4H-Benzo[1,4]thiazin-3-one and 1,1-Dioxo-1,4-dihydro-2H-1λ6-benzo[1,4]thiazin-3-one Derivatives from 1,5-Difluoro-2,4-dinitrobenzene. J. Comb. Chem. 2007, 9, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Sun, M.N.; Liu, G. Substituent Diversity-Directed Synthesis of Indole Derivatives. J. Comb. Chem. 2009, 11, 556–575. [Google Scholar] [CrossRef] [PubMed]
- Sebaoun, L.; Maurizot, V.; Granier, T.; Kauffmann, B.; Huc, I. Aromatic Oligoamide β-Sheet Foldamers. J. Am. Chem. Soc. 2014, 136, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, D.-X.; Wang, M.-X. Oligo-m-aniline Foldamers. Tetrahedron Lett. 2012, 53, 6426–6429. [Google Scholar] [CrossRef]
- Hamuro, Y.; Geib, S.J.; Hamilton, A.D. Novel Folding Patterns in a Family of Oligoanthranilamides: Non-Peptide Oligomers that Form Extended Helical Secondary Structures. J. Am. Chem. Soc. 1997, 119, 10587–10593. [Google Scholar] [CrossRef]
- Issa, Y.M.; Hindawey, A.M.; El-Kholy, A.E.; Issa, R.M. Infrared spectroscopic study of the intermolecular interactions of phenylenediamines with some aromatic nitro compounds. Gazz. Chim. Ital. 1981, 111, 27–34. [Google Scholar]
- Issa, R.M.; Gaber, M.G.; El-Ansary, A.L.; Rizk, H.F. Charge transfer interaction of hydroxy aryl Schiff bases with some aromatic nitrobenzenes. Bull. Soc. Chim. Fr. 1985, 173–176. [Google Scholar]
- Harada, K.-I.; Shimizu, Y.; Kawakami, A.; Norimoto, M.; Fujii, K. Chromatographic Determination of the Absolute Configuration of an Acyclic Secondary Alcohol Using Difluorodinitrobenzene. Anal. Chem. 2000, 72, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Haddoub, R.; Mahé, J.; Marchand, G.; Jacquemin, D.; Edzang, J.A.; Canard, G.; Ferry, D.; Grauby, O.; Ranguis, A.; et al. N-Substituted Azacalixphyrins: Synthesis, Properties, and Self-Assembly. Chem. Eur. J. 2016, 22, 17820–17832. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-J.; Liu, L.-Q.; Ma, M.-L.; Zhao, X.-L.; Liu, Y.A.; Mi, X.-Q.; Jiang, B.; Wen, K. m-Terphenyl-3,3′′-dioxo-derived oxacalixaromatics: Synthesis, structure, and solvent encapsulation in the solid state. Tetrahedron 2013, 69, 3934–3941. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, T.; He, L.; Gong, B. One-Pot Formation of Aromatic Tetraurea Macrocycles. Org. Lett. 2012, 14, 2504–2507. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.L.; Tschaen, B.A. Synthesis of Inherently Chiral Azacalix[4]arenes and Diazadioxacalix[4]arenes. Org. Lett. 2010, 12, 4300–4303. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Mita, T.; Morikawa, O.; Kobayashi, K. Synthesis and disproportionation of ABAC-type oxacalix[4]arenes. Tetrahedron Lett. 2007, 48, 3029–3032. [Google Scholar] [CrossRef]
- Hao, E.; Fronczek, F.R.; Graca, V. Synthesis of oxacalixarene-locked bisporphyrins and higher oligomers. J. Org. Chem. 2006, 71, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Shivanyuk, A.; Far, A.R.; Rebek, J., Jr. Rigid Tetranitroresorcinarenes. Org. Lett. 2002, 4, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Zissimou, G.A.; Berezin, A.A.; Ioannou, T.A.; Manoli, M.; Tsokkou, D.; Theodorou, E.; Hayes, S.C.; Koutentis, P.A. Tetraphenylhexaazaanthracenes: 16π Weakly Antiaromatic Species with Singlet Ground States. Org. Lett. 2015, 17, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Zissimou, G.A.; Constantinides, C.P.; Manoli, M.; Pieridou, G.K.; Hayes, S.C.; Koutentis, P.A. Oxidation of Tetraphenylhexaazaanthracene: Accessing a Scissor Dimer of a 16π Biscyanine. Org. Lett. 2016, 18, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Wudl, F.; Koutentis, P.A.; Weitz, A.; Ma, B.; Strassner, T.; Houk, K.N.; Khan, S.I. Polyazaacenes: New tricks for old dogs. Pure Appl. Chem. 1999, 71, 295–302. [Google Scholar] [CrossRef]
- Koutentis, P.A. Regiospecific synthesis of 5,7-disubstituted quinoxalino[2,3-b]phenazines. ARKIVOC 2002, 6, 175–191. [Google Scholar]
- Riley, A.E.; Mitchell, G.W.; Koutentis, P.A.; Bendikov, M.; Kaszynki, P.; Wudl, F.; Tolbert, S.H. Liquid-Crystalline Phase Behavior in a Zwitterionic Tetraazapentacene. Adv. Funct. Mater. 2003, 13, 531–540. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Koutentis, P.A.; Schatz, J. A DFT Study of the Ground State Multiplicities of Linear vs. Angular Polyheteroacenes. J. Am. Chem. Soc. 2004, 126, 16232–16241. [Google Scholar] [CrossRef] [PubMed]
- Nietzki, R.; Slaboszewicz, J. Ueber eine neue Synthese der Fluorindine. Chem. Ber. 1901, 34, 3727–3732. [Google Scholar] [CrossRef]
- Forbes, E.J.; Tatlow, J.C.; Wragg, R.T. The Unequivocal Syntheses of 2- and 4-trifluoromethylcarbazoles via Diphenyls. Tetrahedron 1960, 8, 73–78. [Google Scholar] [CrossRef]
- Harwood, L.M. “Dry-Column” Flash Chromatography. Aldrichim. Acta 1985, 18, 25–25. [Google Scholar]
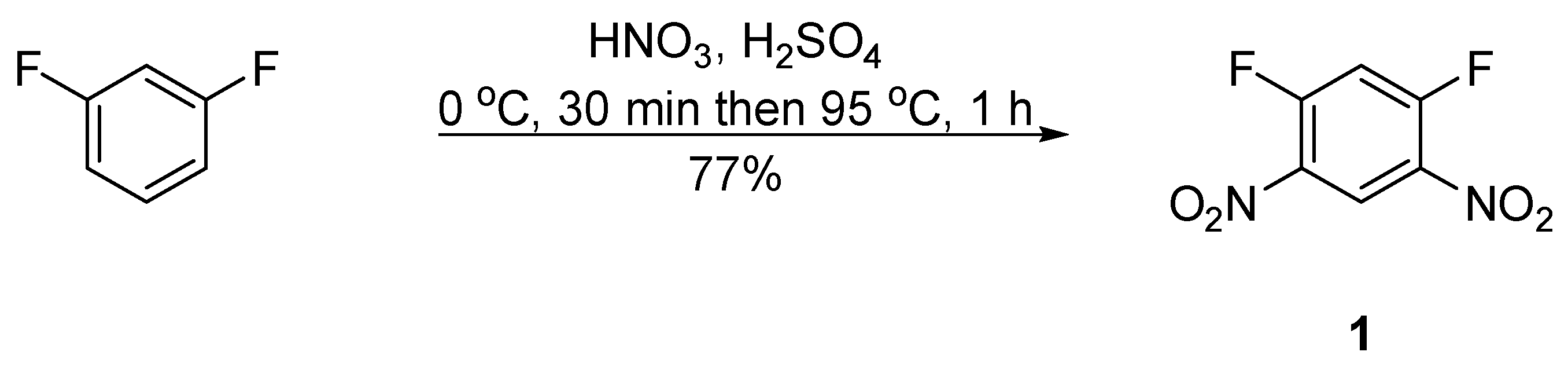
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zissimou, G.A.; Koutentis, P.A. N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine. Molbank 2017, 2017, M967. https://doi.org/10.3390/M967
Zissimou GA, Koutentis PA. N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine. Molbank. 2017; 2017(4):M967. https://doi.org/10.3390/M967
Chicago/Turabian StyleZissimou, Georgia A., and Panayiotis A. Koutentis. 2017. "N1-(5-Fluoro-2,4-dinitrophenyl)-N2-phenyl-4-(trifluoromethyl)benzene-1,2-diamine" Molbank 2017, no. 4: M967. https://doi.org/10.3390/M967





