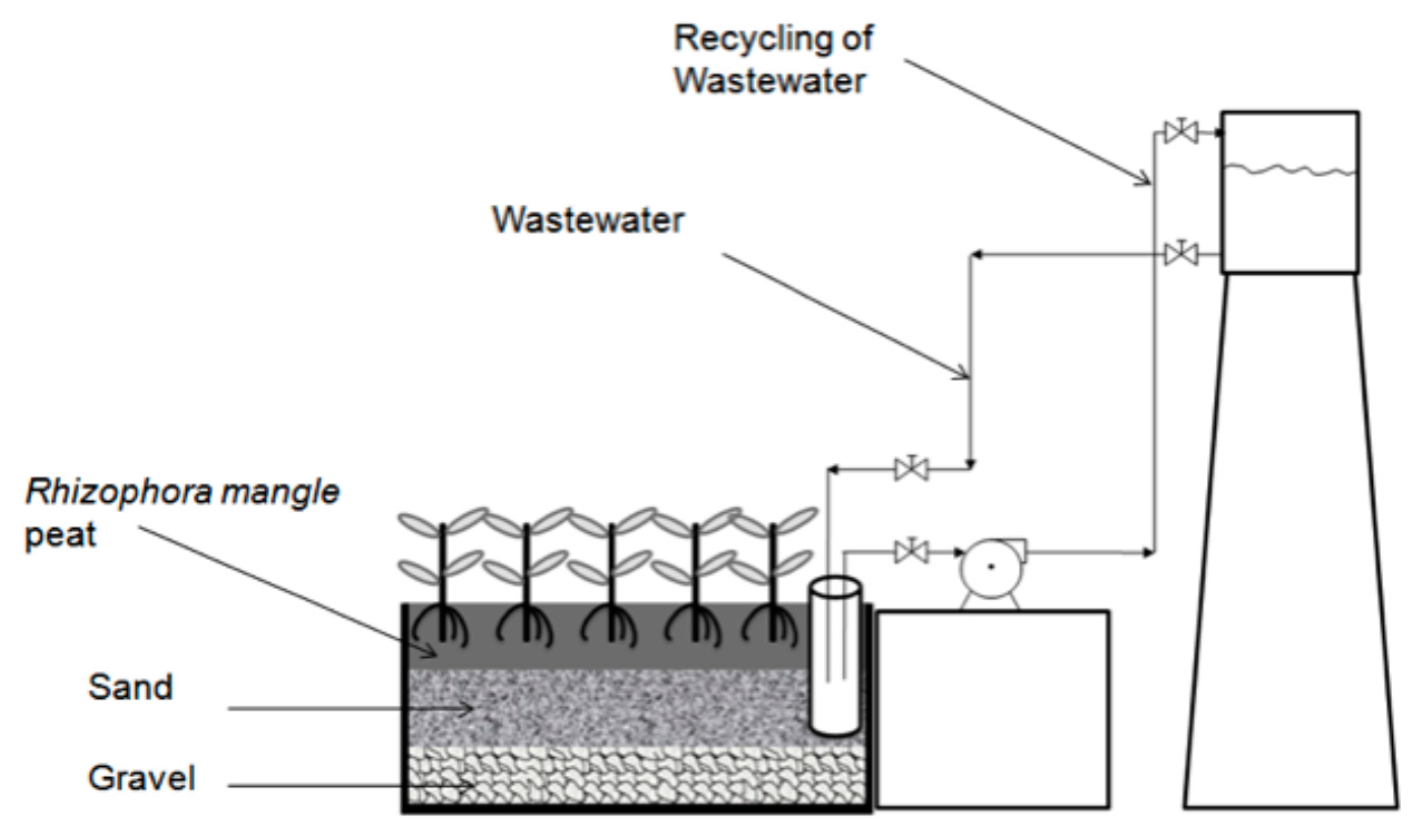
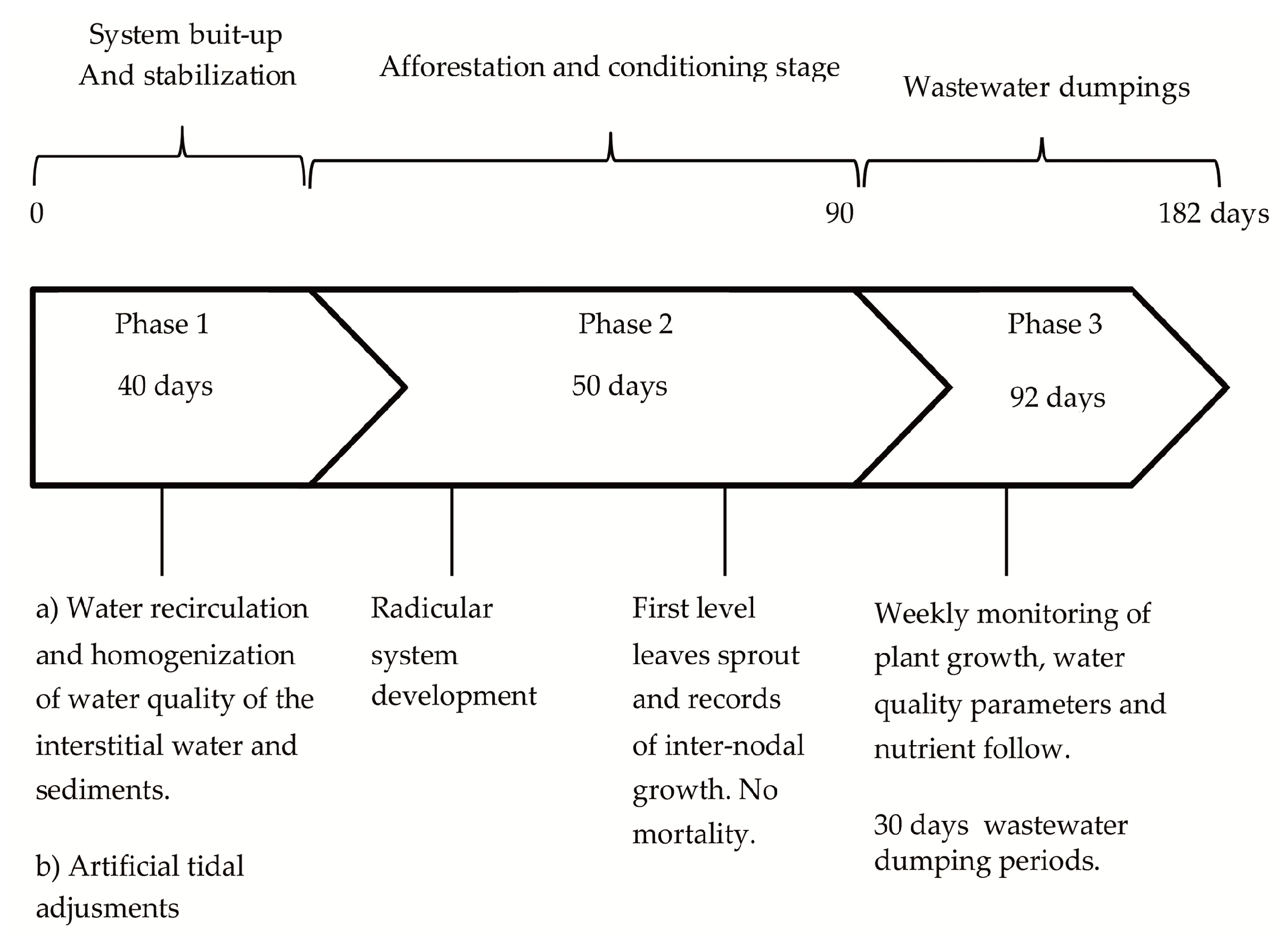
1. Introduction
Coastal environment researchers have highlighted as an important strategy the use of natural or artificial wetland habitats as filters for improving water quality discharges [
1,
2,
3,
4]. The first studies on biofilters were carried out in Germany during the 1950s, although the studies on wetlands as biofilters were initiated by American researchers during the 1960s [
5]. It was not until 1990s that assessments of wetlands as biofilters, with high efficiency as pollutant removers and benefits for the economy, became more common [
6].
Gersberg et al. [
7] compared artificially the nutrient removal efficiency of the cattail (
Typha sp.)—a common wetland plant—and the reed perennial grass (
Phragmites sp.) using sand beds filled with domestic residual water. He reported a high removal rate of nitrogen compounds from freshwater in both cases, but
Typha sp. was the most efficient converter, as it showed a higher biomass production of roots. Martínez-Cruz et al. [
8] made artificial wetland habitats with
Scirpus americanus (enrooted macrophytes) and
Lemna gibba—an aquatic floating plant. In both cases, they obtained significant differences in nutrient removal rates compared with their controls. Habitats with
S.
americanus removed a total of 89%, 86% and 38% of nitrites, ammonia nitrogen and reactive phosphorous from water, respectively, whereas bed filters of
L.
gibba removed 90%, 80%, and 50%, respectively. Consequently, Huang et al. [
9] stated that mangrove forests could be considered a tolerant group of plants for wastewater effluents due to their enormous nutrient demand. Apart from nutrient removal, Bayen [
10] considers that mangroves play a key role in removing and/or degrading pollutants, such as heavy metals and pesticides from estuaries and coastal lagoons.
Robertson et al. [
11] used mangrove species in artificial wetland habitats as biofilters for residual water discharges; they compared the nutrient removal rates (N and P) against a natural forest of
Rhizophora mangle. From their results, they inferred that one-hectare of a semi-intensive or intensive artificial system has the equivalent nutrient removal efficiency of two to twenty hectares of natural forest. In natural environments, Wong et al. [
12] used two intertidal sampling locations of
Kandelia candel and
Aegiceras corniculatum trees in order to test the removal rate of organic carbon and nitrogen; they concluded that these species displayed great biofilter potential as they removed up to 80% of the former compounds which in turn was reflected in biomass and basal area increments. Boonsong et al. [
13] compared and analyzed phosphates removal rates (PO
4−3 and total P) of a mangrove plantation of
Rhizophora sp.,
Avicennia marina,
Bruguiera cylindrica and
Ceriops tagal with a natural mangrove forest receiving residual waters, and found higher values of removal rates than natural forest, therefore concluding that mangrove plantations are ideal biofilters for treating urban wastewaters. Due to this evidence, considerable attention has been directed towards the use of artificially constructed mangrove systems, as they provide an alternative low maintenance, cost-efficient and relative simple method for removal of nutrients and runoff pollutants from water [
9,
14,
15,
16].
The biofilter capacity of mangroves seedlings has also been tested and it seems that this capacity is affected by paramount environmental variables. Wu et al. [
17] demonstrated that seedlings of
Aegiceras corniculatum could remove high amounts of dissolved organic carbon, inorganic and organic ammonia (91%, 98% and 78% respectively), originating from residual water discharges of local areas when salinity was down to zero; however, when salinity was about 30 PSU, the removal efficiency was reduced to 71%, 83% and 56%, respectively. Wu et al. [
18] also tested seedlings of
Kandelia candel in controlled conditions and simulated tidal regimes as nutrient removers of Hong Kong municipal waters. They obtained removal rates of 70.4–76.4%, 75.1–79.1%, 76.2–91.8%, and 47.9–63.4%, of dissolved organic carbon, particulate organic carbon, dissolved inorganic ammonia and total nitrogen, respectively, probably achieving the closest approximations of natural environments removal rates [
18]. Apparently, mangrove seedlings are also good removers of heavy metals as proved by Zhang et al. [
19], who, using a simulated wetland of
Sonneratia apetala, reported an individual removal rate of over 90% of total nitrogen, total phosphorus, copper, zinc, lead and cadmium; final biomass in that system displayed a linear positive correlation with the removal rate of the former elements. Moroyoqui-Rojo et al. [
20] used an experimental silvofishery system planted independently with seedlings of
Rhizophora mangle and
Laguncularia racemosa imitating effluent conditions of aquaculture systems, and found that both plants were good nitrogen removers, while phosphorus removal was not significant.
It is largely known that hydroperiod, redox potential, salinity and temperature influence mangroves’ physiological responses, and that they therefore may affect its removal capacity [
21]. Consequently, we aimed to evaluate the nutrient removal rate and growth responses of
Rhizophora mangle seedlings when submitted to successive wastewater discharges under controlled conditions and simulated tidal regimes. The variation of environmental cues was monitored during the duration of the experiment. Then, the specific response variables assessed were removal rate of nitrogen compounds NH
4+, NO
2−, NO
3−, and phosphorous PO
4−3, and growth in terms of height, diameter and above-ground biomass.
4. Discussion
The percentages of nutrients removal by
R.
mangle seedlings tested here under controlled conditions are very close to the removal capacity displayed by seedlings of other mangrove species, such as
Aegiceras corniculatum at salinity of 30 PSU [
17] (
Table 7), and by seedlings of
Kandelia candel (2.1 PSU) [
18], even though the plants of this study were maintained at a salinity of 26 PSU (
Table 7). Moreover, seedlings of another mangrove species,
Sonneratia apetala removes phosphate at the same magnitude as
R.
mangle [
19]. Boonsong et al. [
13] consider that mangroves of the
Rhizophora genus besides other species are good biofilters with high removal rate of P-PO
4−3, as part of their nutrient absorption metabolism needed for growth [
29] observation that corresponds with the results obtained here.
Rhizophora mangle seems to be a more efficient remover of phosphate and N-ammonia (
Table 7) under oxic conditions when compared to lower removal values (below 50% and 65%) reported by Feller et al. [
30] and Yi Ming et al. [
31] achieved when seedlings thrive through under reduced conditions. In aerobic conditions (i.e., higher redox potential) nutrient removal rate is increased during the nitrification process, as consequence of the concomitant activity of the microbial community that oxidizes ammonia and free nitrogen [
32,
33], thus favoring nitrogen removal from the system.
Ideally, artificially constructed mangrove forests for waste treatment require tidal regimes (low and high tide) in order to generate aerobic and anaerobic conditions in the sediments which promotes nitrification and denitrification processes and facilitation of ammonia removal [
34]. Ammonia is oxidized to NO
2 and then to NO
3 if oxic conditions prevail. However, there are other pathways for which nitrogen compounds are removed under oxic conditions, such as ammonification, ammonia volatilization, nitrification and plants absorption and adsorption processes [
35]. In the absence of a tidal regime, reduced conditions would result in the sediments of artificial mangrove forests under wastewater dumping, with the consequent nitrogen built-up [
36].
Results of the present paper also favor the notion that phosphates are more efficiently removed if oxic conditions are present, as phosphate removal was higher than reported from similar experiments in reduced conditions [
30,
31]. Phosphates are dissolved and retained in the uppermost surface (water–sediment interphase) in oxic conditions, allowing its solubilizing by bacteria associated to the root system, which is a necessary step before plant phosphates assimilation [
19,
37,
38]. Furthermore, we consider that the nutrient removal capacity displayed by
R.
mangle seedlings is also associated to its ability for atmospheric oxygen transference from the aerial to the subterranean roots, facilitating the oxidation of reduced nitrogen compounds (i.e., ammonium-N), byproducts of bacteria nitrification. This mechanism supplies part of the nitrogen demands to the plants [
17,
39]. We noticed that removal rates of DIN and phosphates diminished from the first to the second and form the second to the third dumping (15.6% and 15.9%, respectively). This phenomenon in plants is known as luxury consumption, in other words, excess of nutrients in the immediate environment leads to sediment saturation tampering nutrient incorporation [
29,
40]. This negatively affects plants as has been extensively proved in crop yields and quality. Furthermore, the decreasing removal efficiency of nitrite and nitrate compounds could be attributed to natural reducing conditions occurring in the interstitial water as consequence of the combined effect of bacterial activity and organic matter oxidation. Reduction in the removal efficiency of nitrogen compounds of exposed mangroves over time were recently reported by [
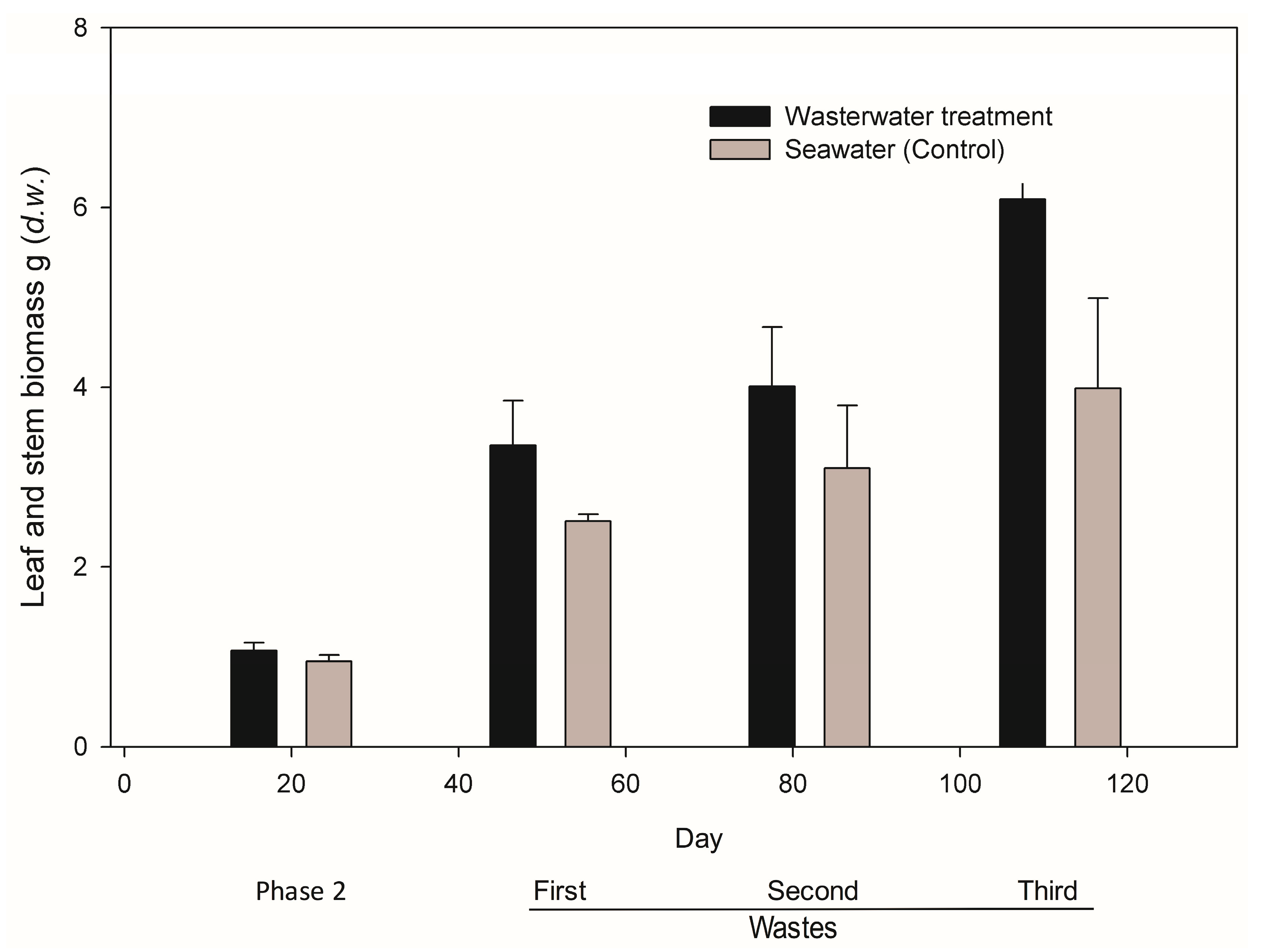
41]. Plant metabolism can also account for the nitrogen removal as they need this compound for their normal growth; in this experiment, biomass in the form of stem and leaves increased significantly between the first and second spill mainly. Similar results are reported for mangroves by Reef et al. (2010).
In the scenario where incoming wastewater continues, nutrient will built-up in sediments until saturation point. At this stage, surrounding waters will enter into eutrophication and the oxidation-reduction capacity in pore-water will turn the environment anoxic (redox potential < −300 mV). Other organic compounds proper to mangrove forests, such as methane, nitrous oxide, ammonia and sulphur are generated, which inhibit enzymatic activity and photosynthesis, situations that are not uncommon in mangroves [
42]. Redox potential below −250 mV favors reduction of sulphates to hydrogen sulfide carbon dioxide and organic matter in sediments are reduced to methane (CH
4). All the former processes affect primary productivity. Tidal regimes associated to anoxic environment, such as places where residence times are high, would not allow oxidative processes in the water-sediment phase. About 20% the plants remove nutrient content and the 80% left is retained in the sediments [
19]. Under constant nutrient inputs, saturation limit is achieved and plants lose their capacity of nutrient assimilation. We believe that biomass increment in leaves and stems observed in
R.
mangle seedlings of the treated group is the consequence of the absorption and assimilation of phosphates and ammonium-N made available between the first and third wastewater spillage. Plants incremented in 45% of their biomass; similar results have been reported for
Bruguiera gymnorrhyza after incrementing its biomass before ammonia and nitrate rises [
43].
Sonneratia apetala incremented its biomass after receiving inputs of municipal untreated waters with high concentrations of nitrogen and phosphorous [
19]. It seems that during our experiment we unintentionally caused nutrient and saturation stress to our experimental subjects—
R.
mangle seedlings—since after the third spill, the nutrient removal rate and the growth rate (height) decreased significantly (29.4%). At this point, we must have achieved nutrient saturation in the interstitial water, propitiating the augmentation of reduced compounds [
43].
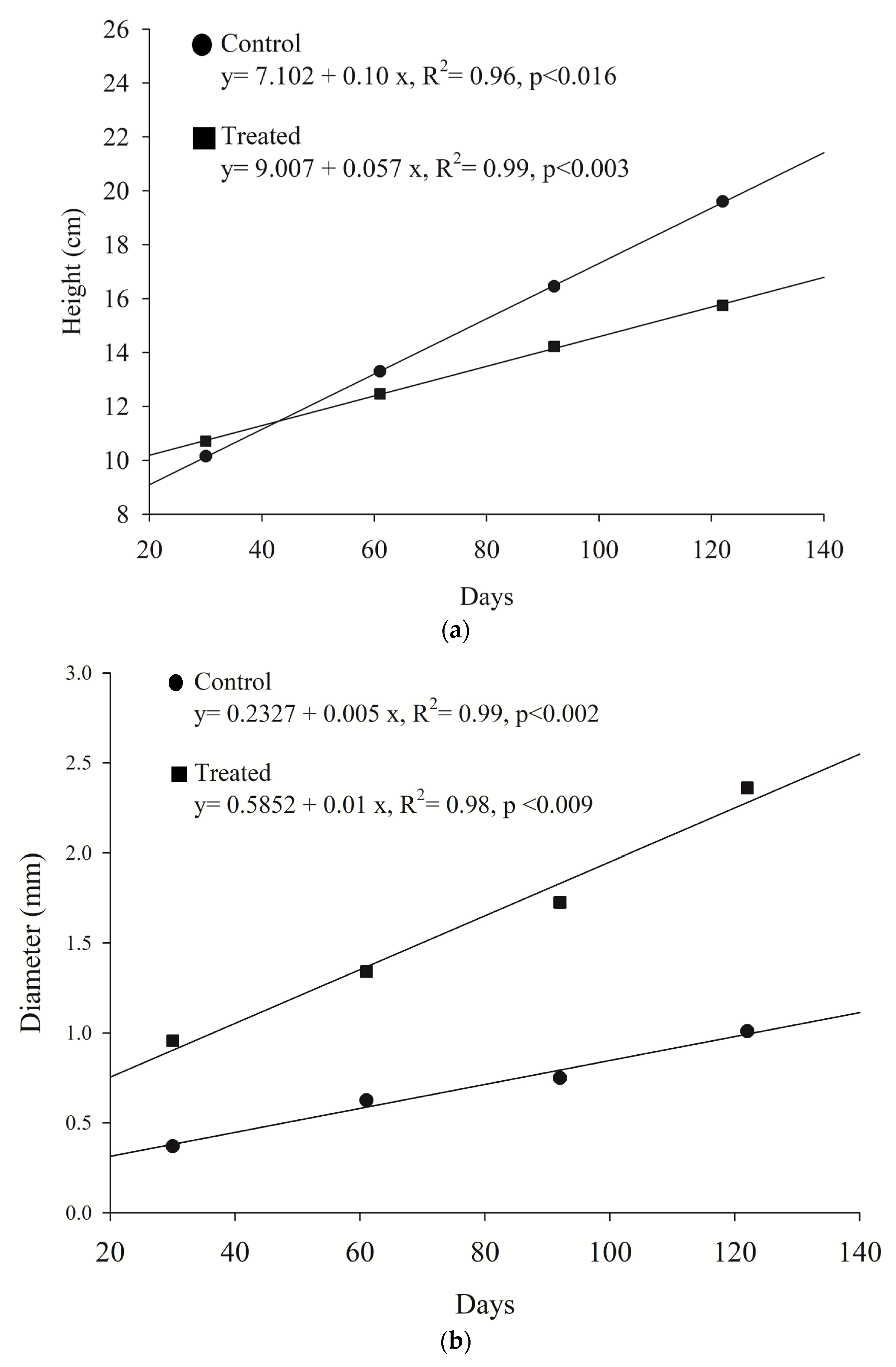
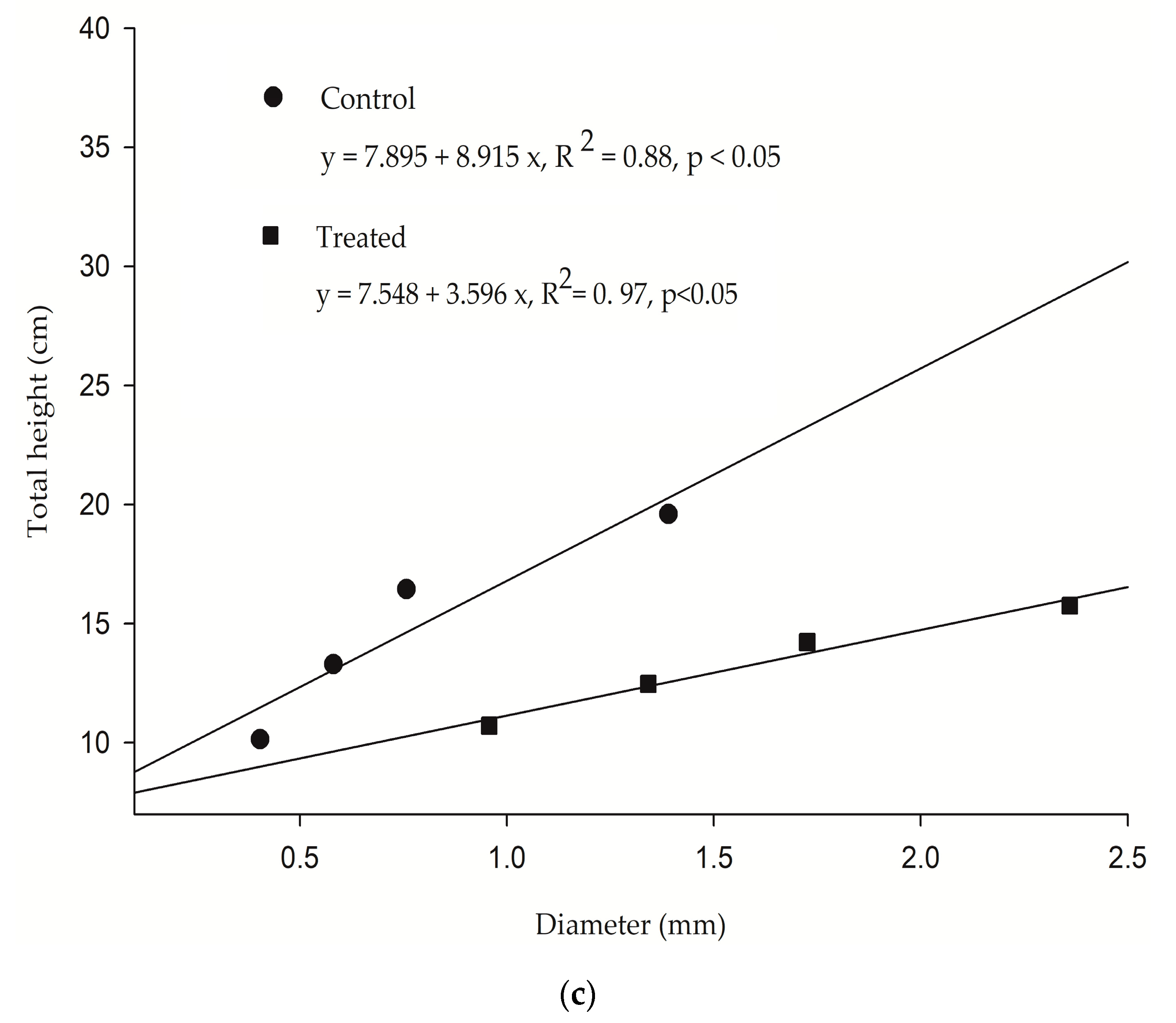
Allometric positive growth between height and girth was verified to either seedlings growing in wastewater or marine water. However, the slope of the curve displayed a 46.9% decrease for the exposed plants (R
2 = 0.97,
p < 0.05), when compared to the untreated group. It is known in plant science that growth increments do not maintain their initial proportions when exposed to constant nutrient uploads (the law of diminishing returns). Asymptotic growth is commonly achieved in this situation and growth stops despite having nutrient availability [
44]. Primary production by the plants also stops; this effect is known as luxury consumption and it is believed that is caused by the imbalance of essential nutrients and toxic byproducts. Vaiphasa et al. [
45] have also suggested that in these conditions, nutrients are accumulated in the aerial structures making the plants susceptible to water deficit [
46].