Evaluation of Conspecific Attraction as a Management Tool across Several Species of Anurans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Sites and Experimental Design
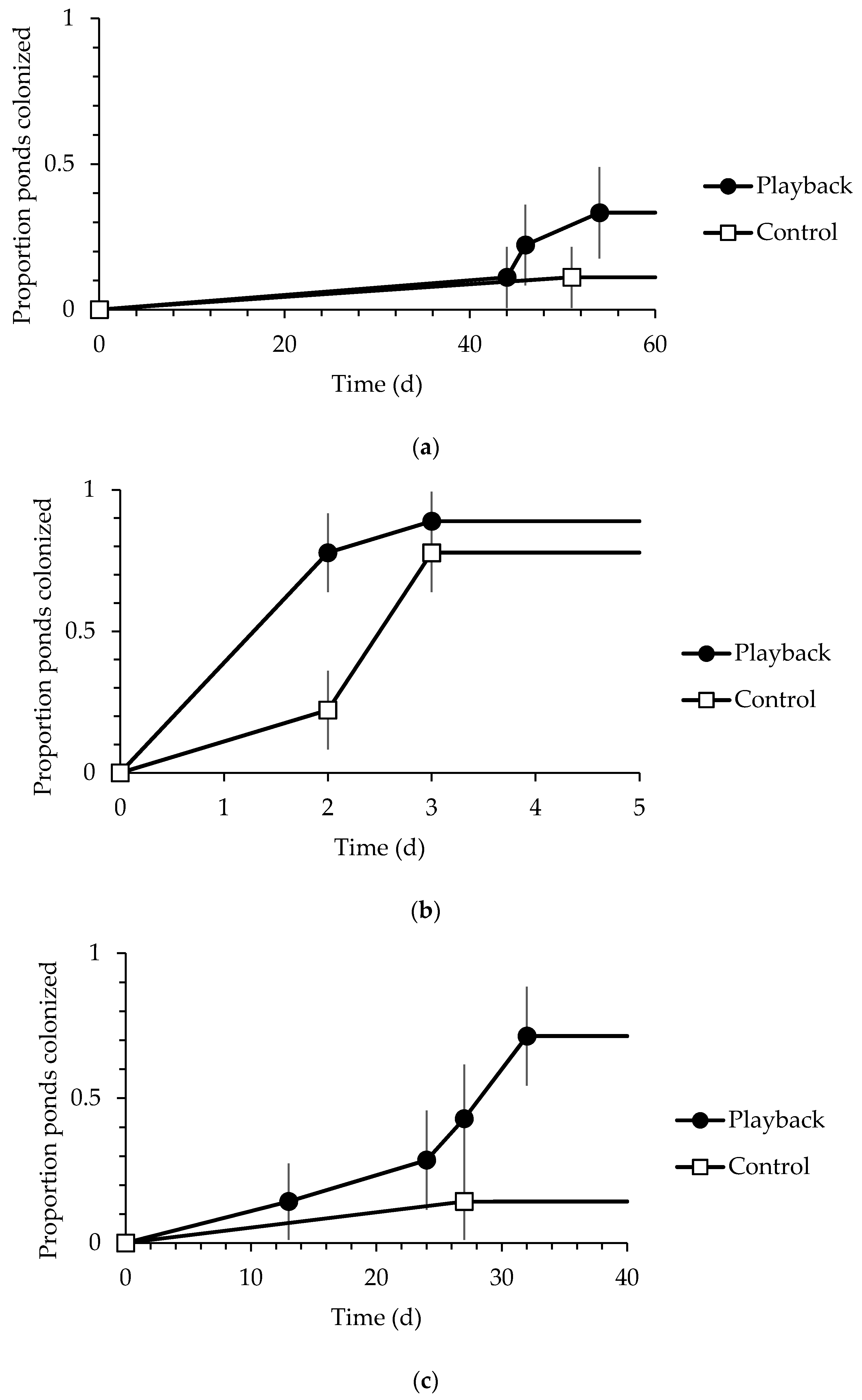
2.2.1. Indiana
2.2.2. Illinois
2.2.3. Arizona
2.3. Monitoring and Data Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Species | Source | Catalog #a | Recordist | General Location |
|---|---|---|---|---|
| Cope’s gray treefrog | Macaulay Library, Cornell Lab of Ornithology | ML181955 | Carl H. Gerhardt | Chatham County, GA |
| Macaulay Library, Cornell Lab of Ornithology | ML183617 | Carl H. Gerhardt | Stoddard County, MO | |
| Macaulay Library, Cornell Lab of Ornithology | ML183750 | Carl H. Gerhardt | Chatham County, GA | |
| Macaulay Library, Cornell Lab of Ornithology | ML185098 | Carl H. Gerhardt | Dent County, MO | |
| Macaulay Library, Cornell Lab of Ornithology | ML176296 | Geoffrey A Keller | Brown County, IN | |
| Wood frog | Macaulay Library, Cornell Lab of Ornithology | ML71895 | Martha J. Fischer | Tompkins County, NY |
| Macaulay Library, Cornell Lab of Ornithology | ML176110 | Geoffrey A. Keller | Brown County, IN | |
| Macaulay Library, Cornell Lab of Ornithology | ML53160 | Steven R. Pantle | Tioga County, NY | |
| Macaulay Library, Cornell Lab of Ornithology | ML130487 | Gregory F. Budney | Tompkins County, NY | |
| The Frogs and Toads of North America Audio CD | NA | Lang Elliott, Carl H. Gerhardt, Carlos Davidson | Unknown | |
| Voices of the Night Audio CD, produced by Cornell Lab of Ornithology | NA | Arthur A. Allen, Peter P. Kellogg | Unknown | |
| Spring peeper | Macaulay Library, Cornell Lab of Ornithology | ML179076 | Carl H. Gerhardt | Chatham County, GA |
| Macaulay Library, Cornell Lab of Ornithology | ML176100 | Geoffrey A. Keller | Brown County, IN | |
| Macaulay Library, Cornell Lab of Ornithology | ML94243 | Wilbur L. Hershberger | Frederick County, MD | |
| Macaulay Library, Cornell Lab of Ornithology | ML190947 | Randolph S. Little | Morris County, NJ | |
| Macaulay Library, Cornell Lab of Ornithology | ML179061 | Carl H. Gerhardt | Chatham County, GA | |
| Macaulay Library, Cornell Lab of Ornithology | ML136511 | Michael J. Andersen | Tompkins County, NY | |
| Macaulay Library, Cornell Lab of Ornithology | ML94263 | Wilbur L. Hershberger | Berkeley County, WV | |
| Green frog | Macaulay Library, Cornell Lab of Ornithology | ML176143 | Geoffrey A. Keller | Brown County, IN |
| Macaulay Library, Cornell Lab of Ornithology | ML138552 | Gregory F. Budney | Hamilton County, NY | |
| Macaulay Library, Cornell Lab of Ornithology | ML163335 | Matthew D. Medler | Tompkins County, NY | |
| The Frogs and Toads of North America Audio CD | NA | Lang Elliott, Carl H. Gerhardt, Carlos Davidson | Unknown | |
| Mexican spadefoot | Personal Recording | NA | Valerie L. Buxton | Cochise County, AZ |
| The Frogs and Toads of North America Audio CD | NA | Lang Elliott, Carl H. Gerhardt, Carlos Davidson | Unknown | |
| Macaulay Library, Cornell Lab of Ornithology | ML193873 | William E. Duellman | Oaxaca, Mexico | |
| Frog and Toad Calls of the Rocky Mountains: Vanishing Voices | NA | Carlos Davidson | Cochise County, AZ | |
| Arizona treefrog | Personal Recording | NA | Valerie L. Buxton | Cochise County, AZ |
| Californiaherps.com | NA | Gary Nafis | Coconino County, AZ | |
| The Frogs and Toads of North America Audio CD | NA | Lang Elliott, Carl H. Gerhardt, Carlos Davidson | Unknown | |
| Frog and Toad Calls of the Rocky Mountains: Vanishing Voices | NA | Carlos Davidson | Coconino County, AZ |
References
- The IUCN Red List of Threatened Species. Version 2017–2. Available online: www.iucnredlist.org (accessed on 16 October 2017).
- Adams, M.J.; Miller, D.A.W.; Muths, E.; Corn, P.S.; Grant, E.H.C.; Bailey, L.L.; Fellers, G.M.; Fisher, R.N.; Sadinski, W.J.; Waddle, H. Trends in amphibian occupancy in the United States. PLoS ONE 2013, 8, e64347. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Beebee, T.J.C.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Cushman, C.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Germano, J.M.; Bishop, P.J. Suitability of amphibians and reptiles for translocation. Conserv. Biol. 2009, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Petranka, J.W.; Holbrook, C.T. Wetland restoration for amphibians: Should local sites be designed to support metapopulations or patchy populations? Restor. Ecol. 2006, 14, 404–411. [Google Scholar] [CrossRef]
- Lehtinen, R.M.; Galatowitsch, S.M. Colonization of restored wetlands by amphibians in Minnesota. Am. Midl. Nat. 2001, 145, 388–396. [Google Scholar] [CrossRef]
- Ahlering, M.A.; Arlt, D.; Betts, M.G.; Fletcher, R.J., Jr.; Nocera, J.J.; Ward, M.P. Research needs and recommendations for the use of conspecific-attraction methods in the conservation of migratory songbirds. Condor 2010, 112, 252–264. [Google Scholar] [CrossRef]
- Stamps, J.A. Conspecifics as cues to territory quality: A preference of juvenile lizards (Anolis aeneus) for previously used territories. Am. Nat. 1987, 129, 629–642. [Google Scholar] [CrossRef]
- Ward, M.P.; Schlossberg, S. Conspecific attraction and the conservation of territorial songbirds. Conserv. Biol. 2004, 18, 519–525. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr. Emergent properties of conspecific attraction in fragmented landscapes. Am. Nat. 2006, 168, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Kress, S. The use of decoys, sound recordings and gull control for re-establishing a tern colony in Maine. Colonial Waterbirds 1983, 6, 185–196. [Google Scholar] [CrossRef]
- Furmankiewicz, J.; Ruczński, I.; Urban, R.; Jones, G. Social calls provide tree dwelling bats with information about the location of conspecifics at roosts. Ethology 2011, 117, 480–489. [Google Scholar] [CrossRef]
- Lecchini, D.; Shima, J.; Banaigs, B.; Galzin, R. Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 2005, 143, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Stamps, J.A. Conspecific attraction and aggregation in territorial species. Am. Nat. 1988, 131, 329–347. [Google Scholar] [CrossRef]
- Gerhardt, H.C.; Klump, G.M. Phonotactic responses and selectivity of barking treefrogs (Hyla gratiosa) to chorus sounds. J. Comp. Physiol. A 1988, 163, 795–802. [Google Scholar] [CrossRef]
- Nocera, J.J.; Forbes, G.J.; Giraldeau, L.A. Inadvertent social information in breeding site selection of natal dispersing birds. Proc. R. Soc. Lond. B 2006, 273, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.C.; Huber, F. Acoustic Communications in Insects and Anurans: Common Problems and Diverse Solutions; University of Chicago Press: Chicago, IL, USA, 2002; ISBN 9780226288338. [Google Scholar]
- Gerhardt, H.C.; Bee, M.A. Recognition and localisation of acoustic signals. In Hearing and Sound Communication in Amphibians, Narins, P.M., Feng, A.S., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2007; pp. 113–146. [Google Scholar]
- Oldham, R.S. Orienting mechanisms of the green frog, Rana clamitans. Ecology 1967, 48, 477–491. [Google Scholar] [CrossRef]
- James, M.L.; Stockwell, M.P.; Clulow, J.; Clulow, S.; Mahony, M.J. Investigating behavior for conservation goals: Conspecific call playback can be used to alter amphibian distributions within ponds. Biol. Conserv. 2015, 192, 287–293. [Google Scholar] [CrossRef]
- Bee, M.A. Selective phonotaxis by male wood frogs (Rana sylvatica) to the sound of a chorus. Behav. Ecol. Sociobiol. 2007, 61, 955–966. [Google Scholar] [CrossRef]
- Swanson, E.M.; Tekmen, S.M.; Bee, M.A. Do female frogs exploit inadvertent social information to locate breeding aggregations. Can. J. Zool. 2007, 85, 921–932. [Google Scholar] [CrossRef]
- Christie, K.; Schul, J.; Feng, A.S. Phonotaxis to male’s calls embedded within a chorus by female gray treefrogs, Hyla versicolor. J. Comp. Physiol. A 2010, 196, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Buxton, V.L.; Ward, M.P.; Sperry, J.H. Use of chorus sounds for location of breeding habitat in 2 species of anuran amphibian. Behav. Ecol. 2015, 26, 1011–1018. [Google Scholar] [CrossRef]
- Denton, R.D.; Richter, S.C. Amphibian communities in natural and constructed ridge top wetlands with implications for wetland construction. J. Wildl. Manag. 2013, 77, 886–896. [Google Scholar] [CrossRef]
- Dodd, K.C. Frogs of the United States and Canada; John Hopkins University Press: Baltimore, MD, USA, 2013; ISBN 9781421406336. [Google Scholar]
- Rannap, R.; Lõhmus, A.; Briggs, L. Restoring ponds for amphibians: A success story. Hydrobiologia 2009, 634, 87–95. [Google Scholar] [CrossRef]
- Ward, M.P.; Semel, B.; Jablonski, C.; Deutsch, C.; Giammaria, V.; Miller, S.B.; McGuire, B.M. Consequences of using conspecific attraction in avian conservation: A case study of endangered colonial waterbirds. Waterbirds 2011, 34, 476–480. [Google Scholar] [CrossRef]
- Andrews, J.E.; Brawn, J.D.; Ward, M.P. When to use social cues: Conspecific attraction at newly created grasslands. Condor 2015, 117, 297–305. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr. Species interactions and population density mediate the use of social cues for habitat selection. J. Anim. Ecol. 2007, 76, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rivera, C.C. Call Timing Interactions, Aggressive Behavior, and the Role of Acoustic Cues in Chorus Formation in Treefrogs. Ph.D. Dissertation, University of Missouri-Columbia, Columbia, MO, USA, 2008. [Google Scholar]
- Mims, M.C.; Hauser, L.; Goldberg, C.S.; Olden, J.D. Genetic differentiation, isolation-by-distance, and indications of a metapopulation of the Arizona treefrog (Hyla wrightorum) in an isolated portion of its range. PLoS ONE 2016, 11, e0160655. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.B. Spatial relationships of male green frogs (Rana clamitans) throughout the activity season. Am. Midl. Nat. 2002, 148, 394–400. [Google Scholar] [CrossRef]
- Albrecht-Mallinger, D.J.; Bulluck, L.P. Limited evidence for conspecific attraction in a low-density population of a declining songbird, the Golden-winged Warbler (Vermivora chrysoptera). Condor 2016, 118, 451–462. [Google Scholar] [CrossRef]
- Ahlering, M.A.; Faaborg, J. Avian habitat management meets conspecific attraction: If you build it, will they come? Auk 2006, 123, 301–312. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Wilbur, H.M. Putting declining amphibian populations in perspective: Natural fluctuations and human impacts. Herpetologica 1994, 50, 65–84. [Google Scholar]
- Virzi, T.; Boulton, R.L.; Davis, M.J.; Gilroy, J.J.; Lockwood, J.L. Effectiveness of artificial song playback on influencing the settlement decisions of an endangered resident grassland passerine. Condor 2012, 114, 846–855. [Google Scholar] [CrossRef]
- Diego-Rasilla, F.J.; Luengo, R.M. Heterospecific call recognition and phonotaxis in the orientation behavior of the marbled newt, Triturus marmoratus. Behav. Ecol. Sociobiol. 2004, 55, 556–560. [Google Scholar] [CrossRef]
- Trillo, P.A.; Bernal, X.E.; Caldwell, M.S.; Halfwerk, W.H.; Wessel, M.O.; Page, R.A. Collateral damage or a shadow of safety? The effects of signaling heterospecific neighbours on the risks of parasitism and predation. Proc. R. Soc. B 2016, 283, 20160343. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, J.; Forsman, J.T.; Mönkkönen, M.; Thomson, R.L. Information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 2007, 88, 1622–1633. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Sieving, K.E. Social-information use in heterogeneous landscapes: A prospectus. Condor 2010, 112, 225–234. [Google Scholar] [CrossRef]
- Seigel, R.A.; Dodd, C.K., Jr. Translocation of amphibians: Proven management method or experimental technique? Conserv. Biol. 2002, 16, 552–554. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Nat. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [PubMed]
| Species | Breeding Habitat | Breeding Period | Study Site |
|---|---|---|---|
| Hyla chrysoscelis | Seasonal | Prolonged | IL |
| Lithobates clamitans | Permanent | Prolonged | IL |
| Lithobates sylvatica | Seasonal | Explosive | IN |
| Pseudacris crucifer | Ephemeral–Permanent | Prolonged | IL |
| Spea multiplicata | Ephemeral | Explosive | AZ |
| Hyla wrightorum | Permanent a | Explosive | AZ |
| Species | Site | Adults Observed | Fisher’s Exact Test | Latency to Colonization | Eggs Observed | Fisher’s Exact Test | Latency to Colonization | ||
|---|---|---|---|---|---|---|---|---|---|
| % Playback | % Control | % Playback | % Control | ||||||
| Hyla chrysoscelis | Indiana ǂ | 100 (9/9) | 33 (3/9) | p = 0.009 | p = 0.001 | 78 (7/9) | 11 (1/9) | p = 0.015 | p = 0.005 |
| Illinois 1 | 33 (3/9) | 11 (1/9) | p = 0.578 | p = 0.259 | 22 (2/9) | 22 (2/9) | p = 1.000 | ||
| Lithobates clamitans | Indiana | 0 | 0 | 0 | 0 | ||||
| Lithobates sylvatica | Indiana | 89 (8/9) | 78 (7/9) | p = 1.000 | p = 0.085 | 67 (6/9) | 44 (4/9) | p = 0.637 | p = 0.463 |
| Pseudacris crucifer | Illinois | 11 (1/9) | 0 | p = 1.000 | 0 | 0 | |||
| Spea multiplicata | Arizona | 71 (5/7) | 14 (1/7) | p = 0.103 | p = 0.040 | 57 (4/7) | 0 | p = 0.069 | |
| Hyla wrightorum | Arizona | 0 | 0 | 13 (1/8) | 0 | p = 1.000 | |||
| Anaxyrus americanus | Indiana ǂ | 0 | 0 | 0 | 0 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buxton, V.L.; Ward, M.P.; Sperry, J.H. Evaluation of Conspecific Attraction as a Management Tool across Several Species of Anurans. Diversity 2018, 10, 6. https://doi.org/10.3390/d10010006
Buxton VL, Ward MP, Sperry JH. Evaluation of Conspecific Attraction as a Management Tool across Several Species of Anurans. Diversity. 2018; 10(1):6. https://doi.org/10.3390/d10010006
Chicago/Turabian StyleBuxton, Valerie L., Michael P. Ward, and Jinelle H. Sperry. 2018. "Evaluation of Conspecific Attraction as a Management Tool across Several Species of Anurans" Diversity 10, no. 1: 6. https://doi.org/10.3390/d10010006





