Post-Glacial Spatial Dynamics in a Rainforest Biodiversity Hot Spot
Abstract
:1. Introduction
1.1. Nothofagus moorei and Highland Temperate Rainforests
1.2. Elaeocarpus grandis and Lowland Subtropical Rainforests
1.3. Aims
2. Results and Discussion
2.1. Post-Glacial Changes in Modelled Climate Suitability
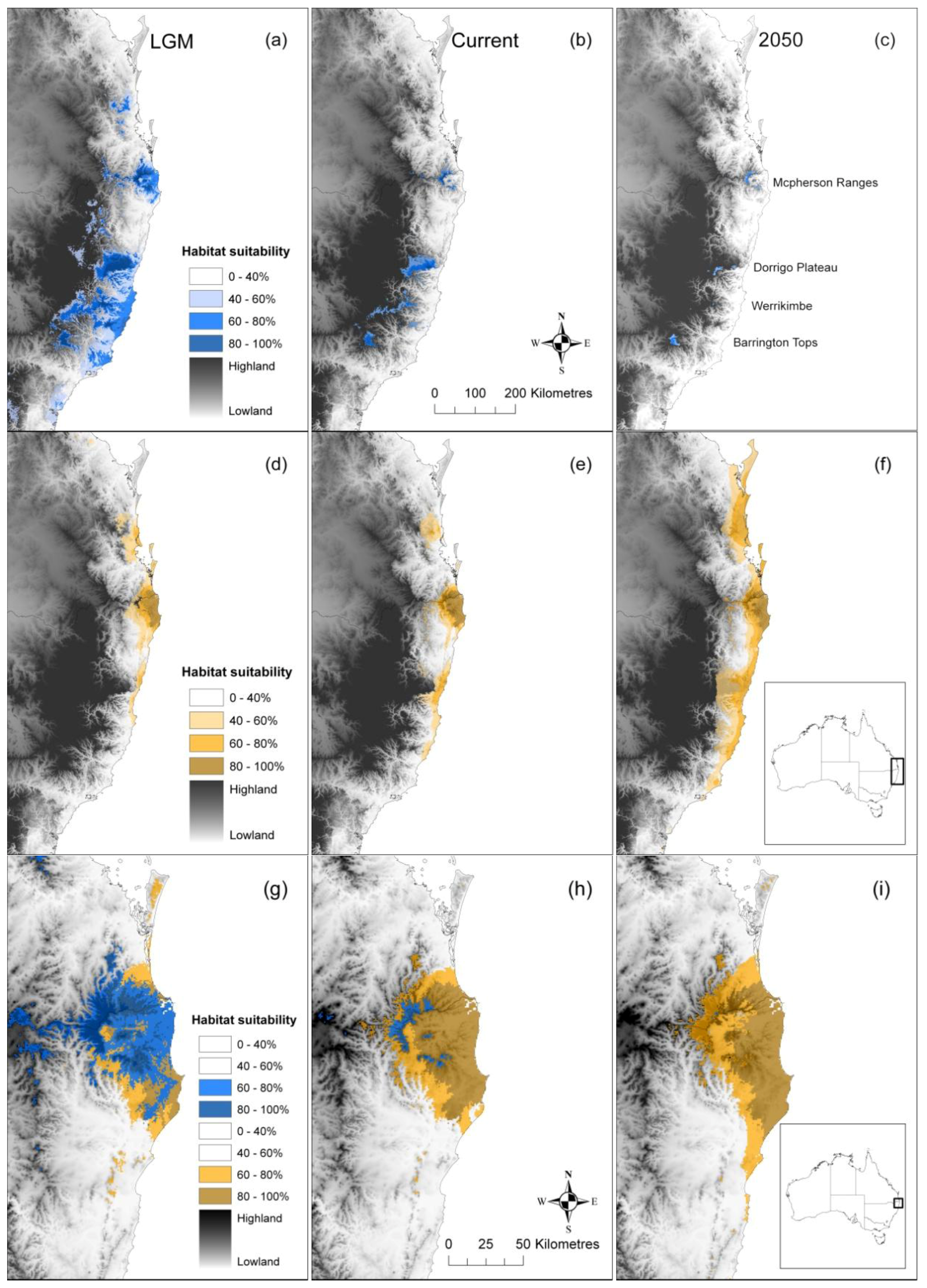
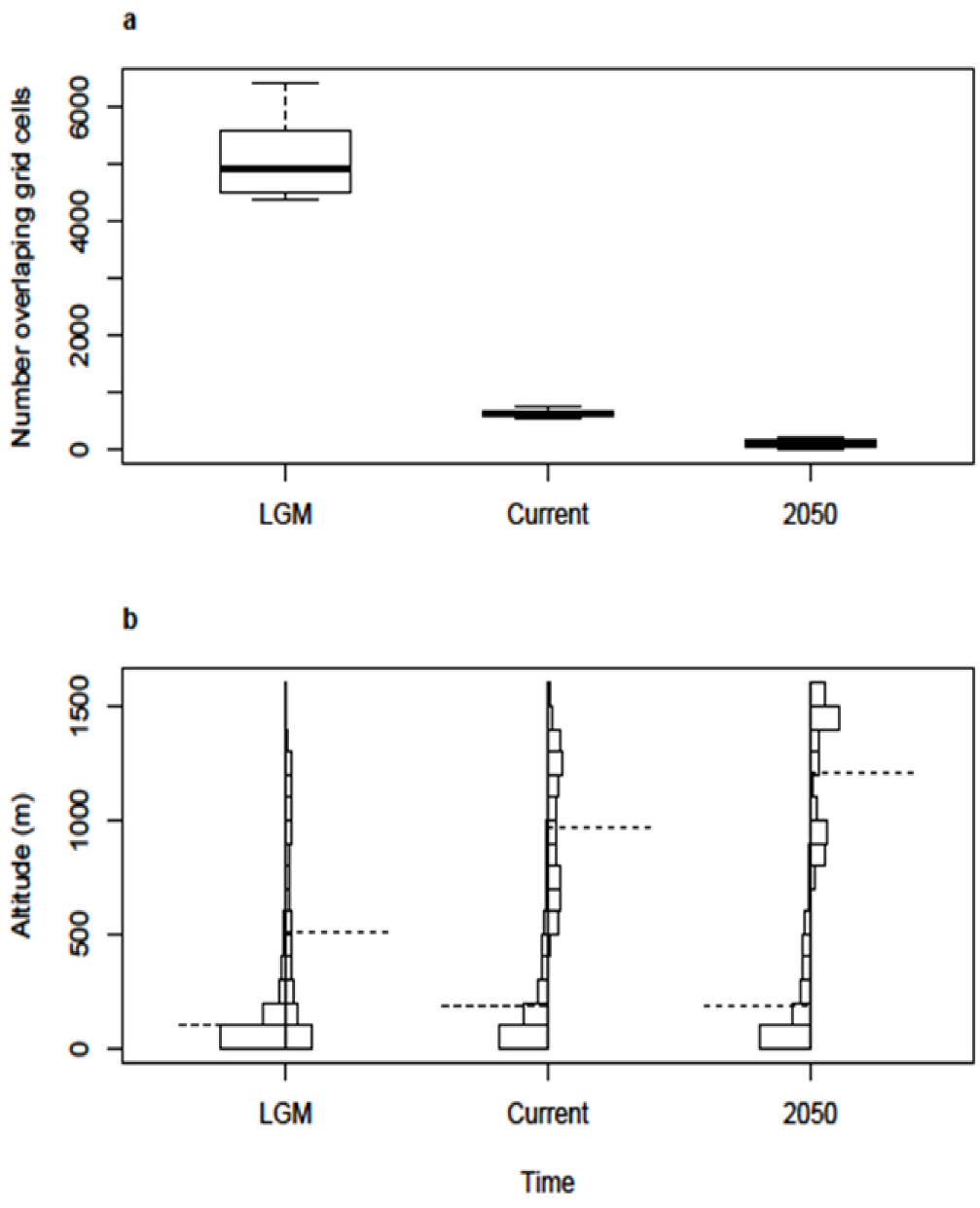
2.2. Spatial and Altitudinal Overlap
2.3. Discussion

3. Experimental Section
4. Conclusion
Acknowledgements
References
- Webb, L.J.; Tracey, J.G. Australian Rainforests: Patterns and Change. In Ecological Biogeography of Australia; Keast, A., Ed.; The Hague: Netherlands, 1981; pp. 607–694. [Google Scholar]
- Hilbert, D.W.; Graham, A.; Hopkins, M.S. Glacial and interglacial refugia within a long-term rainforest refugium: The wet tropics bioregion of northeast Queensland, Australia. Palaeogeogr. Palaeocl. 2007, 251, 104–118. [Google Scholar] [CrossRef]
- VanDerWal, J.; Shoo, L.P.; Williams, S.E. New approaches to understanding late quaternary climate fluctuations and refugial dynamics in Australian wet tropical rain forests. J. Biogeogr. 2009, 36, 291–301. [Google Scholar] [CrossRef]
- Burbidge, N.T. The phytogeography of the Australian region. Aust. J. Bot. 1960, 8, 75–211. [Google Scholar] [CrossRef]
- Mellick, R.; Lowe, A.; Allen, C.D.; Hill, R.S.; Rossetto, M. Palaeodistribution modelling and genetic evidence highlight differential post-glacial range shifts of a rain forest conifer distributed across a latitudinal gradient. J. Biogeogr. 2012, 39, 2292–2302. [Google Scholar] [CrossRef]
- Mellick, R.; Lowe, A.; Rossetto, M. Consequences of long- and short-term fragmentation on the genetic diversity and differentiation of a late successional rainforest conifer. Aust. J. Bot. 2011, 59, 351–362. [Google Scholar]
- Rossetto, M.; Kooyman, R. The tension between dispersal and persistence regulates the current distribution of rare palaeo-endemic rain forest flora: A case study. J. Ecol. 2005, 93, 906. [Google Scholar] [CrossRef]
- Rossetto, M.; Kooyman, R.; Sherwin, W.; Jones, R. Dispersal limitations, rather than bottlenecks or habitat specificity, can restrict the distribution of rare and endemic rainforest trees. Am. J. Bot. 2008, 95, 321–329. [Google Scholar] [CrossRef]
- Rossetto, M.; Crayn, D.; Ford, A.; Mellick, R.; Sommerville, K. The influence of environment and life-history traits on the distribution of genes and individuals: A comparative study of 11 rainforest trees. Mol. Ecol. 2009, 18, 1422–1438. [Google Scholar] [CrossRef]
- Laidlaw, M. Monitoring for climate driven floristic shift in Australian subtropical rainforest. Australas Plant Conserv. 2012, 20, 16–17. [Google Scholar]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2011, 16, 488–495. [Google Scholar] [CrossRef]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trend Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Kershaw, A.P.; McKenzie, G.M.; Porch, N.; Roberts, R.G.; Brown, J.; Heijnis, H.; Orr, M.L.; Jacobsen, G.; Newallt, P.R. A high-resolution record of vegetation and climate through the last glacial cycle from Caledonia Fen, south eastern highlands of Australia. J. Quat. Sci. 2007, 22, 481–500. [Google Scholar] [CrossRef]
- Rossetto, M. From populations to communities: Understanding changes in rainforest diversity through the integration of molecular, ecological and environmental data. Telopea 2008, 12, 47–58. [Google Scholar]
- Kooyman, R.; Rossetto, M.; Cornwell, W.; Westoby, M. Phylogenetic tests of community assembly across regional to continental scales in tropical and subtropical rain forests. Global Ecol. Biogeogr. 2011, 20, 707–716. [Google Scholar] [CrossRef]
- Hill, R.S.; Dettman, M.E. Origin and Diversification of the Genus Nothofagus. In The Ecology and Biogeography of Nothofagus Forest; Veblen, T.T., Hill, R.S., Eds.; Yale University Press: New Haven, CT, USA, 1996; pp. 11–24. [Google Scholar]
- Hill, R.S. The History of Selected Australian Taxa. In History of the Australian Vegetation:Cretaceous to Recent; Hill, R.S., Ed.; Cambridge University Press: Cambridge, UK, 1994; pp. 390–419. [Google Scholar]
- Quinn, C.J.; Price, R.A. Phylogeny of the Southern Hemisphere conifers. Acta Hortic. 2003, 615, 129–136. [Google Scholar]
- Hill, R.S. Nothofagus: Evolution from a southern perspective. Trend Ecol. Evol. 1992, 7, 190–194. [Google Scholar] [CrossRef]
- Hill, R.S. Biogeography, evolution and palaeoecology of Nothofagus. (Nothofagaceae): The contribution of the fossil record. Aust. J. Bot. 2001, 49, 321–332. [Google Scholar] [CrossRef]
- Read, J.; Hill, R.S.; Hope, G.S.; Carpenter, R.J. The contrasting biology of tropical versus temperate Nothofagus species and its relevance to interpretations of Cenozoic rainforest history in southeast Australia. Terra Australis. 2010, 32, 257–274. [Google Scholar]
- Read, J.; Hope, G.S. Foliar frost-resistance of some evergreen tropical and extratropical Australasian Nothofagus species. Aust. J. Bot. 1989, 37, 361–373. [Google Scholar] [CrossRef]
- Dodson, J.R. Mire development and environmental change, Barrington Tops, New South Wales, Australia. Quat. Res. 1987, 27, 73–81. [Google Scholar] [CrossRef]
- Hutley, L.B.; Doley, D.; Yates, D.J.; Boonsaner, A. Water balance of an Australian subtropical rainforest at altitude: The ecological and physiological significance of intercepted cloud and fog. Aust. J. Bot. 1997, 45, 311–329. [Google Scholar] [CrossRef]
- Franks, A.J.; Bergstrom, D.M. Corticolous bryophytes in microphyll fern forests of south-east Queensland: Distribution on Antarctic Beech (Nothofagus moorei). Austral. Ecol. 2000, 25, 386–393. [Google Scholar] [CrossRef]
- Bale, C.L.; Williams, J.B. Composition and classification of Nothofagus moorei communities in northern New South Wales. Aust. Syst. Bot. 1993, 6, 429–440. [Google Scholar] [CrossRef]
- Hill, R.S.; Jordan, G.J. The evolutionary history of Nothofagus (Nothofagaceae). Aust. Syst.Bot. 1993, 6, 111–126. [Google Scholar] [CrossRef]
- Linder, P.H.; Crisp, M.D. Nothofagus and pacific biogeography. Cladistics 1995, 11, 5–32. [Google Scholar] [CrossRef]
- Premoli, A.C.; Steinke, L. Genetics of sprouting: Effects of long-term persistence in fire-prone ecosystems. Mol. Ecol. 2008, 3827–3835. [Google Scholar] [CrossRef]
- Schultz, L. Conservation genetics of a Gondwana relict rainforest tree, Nothofagus moorei (f.Muell.) Krasser. PhD Thesis, Queensland University of Technology, Brisbane, Australia, 2008. [Google Scholar]
- Taylor, K.J.; Lowe, A.J.; Hunter, R.J.; Ridgway, T.; Gresshoff, P.M.; Rossetto, M. Genetic diversity and regional identity in the Australian remnant Nothofagus moorei. Aust. J. Bot. 2005, 53, 437–444. [Google Scholar] [CrossRef]
- Coode, M.J.E. Elaeocarpus. in Australia and New Zealand. Kew Bull. 1984, 39, 509–586. [Google Scholar] [CrossRef]
- Rossetto, M.; Jones, R.; Hunter, J. Genetic effects of rainforest fragmentation in an early successional tree (Elaeocarpus grandis). Heredity 2004, 93, 610–618. [Google Scholar] [CrossRef]
- Webb, L.J.; Tracey, J.G.; Williams, W.T. A floristic framework of Australian rainforests. Aust. J. Ecol. 1984, 9, 169–198. [Google Scholar] [CrossRef]
- Harden, G.J. Flora of NewSouth. Wales; University of NSW press: Sydney, Australia, 1990. [Google Scholar]
- Landcare, B.S.R. Subtropical Rainforest Restoration: A practical Manual and Data Source for Landcare Groups, Land Managers and Rainforest Regenerators, 2nd ed; Big Scrub Rainforest Landcare Group: Lismore, Australia, 2005. [Google Scholar]
- Crayn, D.M.; Rossetto, M.; Maynard, D.J. Molecular phylogeny and dating reveals an Oligo-Miocene radiation of dry-adapted shrubs (former Tremandraceae) from rainforest tree progenitors (Elaeocarpaceae) in Australia. Am. J. Bot. 2006, 93, 1328. [Google Scholar] [CrossRef]
- Pole, M. The New Zealand flora-entirely long-distance dispersal? J. Biogeogr. 1994, 21, 625–635. [Google Scholar] [CrossRef]
- Winkworth, R.C.; Wagstaff, S.J.; Glenny, D.; Lockhart, P.J. Plant dispersal N.E.W.S. from New Zealand. Trends Ecol. Evol. 2002, 17, 514–520. [Google Scholar] [CrossRef]
- Rozefelds, A.C.; Christophel, D.C. Elaeocarpus (Elaeocarpaceae) endocarps from the Oligo-Miocene of eastern Australia. Pap. Proc. R. Soc. Tasman. 1996, 130, 41–48. [Google Scholar]
- Rozefelds, A.C.; Christophel, D.C. Cenozoic Elaeocarpus (Elaeocarpaceae) fruits from Australia. Alcheringa 2002, 26. [Google Scholar]
- Rossetto, M.; Crayn, D.; Ford, A.; Ridgeway, P.; Rymer, P. The comparative study of range-wide genetic structure across related, co-distributed rainforest trees reveals contrasting evolutionary histories. Aust. J. Bot. 2007, 55, 416–424. [Google Scholar] [CrossRef]
- Rossetto, M.; Jones, R.C.; McNally, J. Isolation of microsatellite loci from a rainforest tree, Elaeocarpus grandis (Elaeocarpaceae), and amplification across closely related taxa. Mol. Ecol.Notes 2002, 2, 179–181. [Google Scholar] [CrossRef]
- Office of Environment and Heritage’s vegetation survey database (YETI). Available online: http://www.environment.nsw.gov.au/research/VISplot.htm) (accessed on 4 January 2013).
- Atlas of Living Australia databases. Available online: http://www.ala.org.au/) (accessed on 4 January 2013).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Busby, J.R. Bioclim-a bioclimate analysis and prediction system. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- PMIP2 website. Available online: http://pmip2.lsce.ipsl.fr/) (accessed on 4 January 2013).
- Solomon, S.; Qin, D.; Manning, M.; Marquis, M.; Averyt, K.; Tignor, M.M.B.; Miller, H.L., Jr.; Chen, Z. Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Nakicenovic, N.; Alcamo, J.; Davis, G.; de Vries, B.; Fenhann, J.; Gaffin, S.; Gregory, K.; Grubler, A.; Jung, T.Y.; Kram, T.; et al. Special Report on Emissions Scenarios : A Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Raupach, M.R.; Marland, G.; Ciais, P.; Le Quéré, C.; Canadell, J.G.; Klepper, G.; Field, C.B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. USA 2007, 104, 10288–10293. [Google Scholar]
- Climate Model Inter-comparison Project dataset. Available online: https://www.esg.llnl.gov:8443/home/publicHomePage.do (accessed on 4 January 2013).
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C++. The Art of Scientific Computing, 2nd ed; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol.Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- MaxEnt 3.3.3e software. Available online: http://www.cs.princeton.edu/~schapire/maxent/ (accessed on 4 January 2013).
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Saatchi, S.; Buermann, W.; Ter Steege, H.; Mori, S.; Smith, T.B. Modeling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sens. Environ. 2008, 112, 2000–2017. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- WorldClim website. Available online: http://www.worldclim.org/ (accessed on 4 January 2013).
- R Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2012.
- Christophel, D.C. Evolution of the Australian flora. Plant Syst. Evol. 1989, 63–78. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mellick, R.; Wilson, P.D.; Rossetto, M. Post-Glacial Spatial Dynamics in a Rainforest Biodiversity Hot Spot. Diversity 2013, 5, 124-138. https://doi.org/10.3390/d5010124
Mellick R, Wilson PD, Rossetto M. Post-Glacial Spatial Dynamics in a Rainforest Biodiversity Hot Spot. Diversity. 2013; 5(1):124-138. https://doi.org/10.3390/d5010124
Chicago/Turabian StyleMellick, Rohan, Peter D. Wilson, and Maurizio Rossetto. 2013. "Post-Glacial Spatial Dynamics in a Rainforest Biodiversity Hot Spot" Diversity 5, no. 1: 124-138. https://doi.org/10.3390/d5010124



