Effect of Disturbance Regime on Alpha and Beta Diversity of Rock Pools
Abstract
:1. Introduction
2. Experimental
2.1. Study Site
2.2. Experimental Design
2.3. Data Collection
2.4. Statistical Analyses
2.4.1. Alpha Diversity
2.4.2. Beta Diversity
3. Results and Discussion
| Phylum | Class | Taxa |
|---|---|---|
| Bacillariophyta | Bacillariophyceae | Berkeleya rutilans |
| Coscinodiscophyceae | Melosira sp. | |
| Chlorophyta | Ulvophyceae | Ulvaria obscura |
| Phaeophyta | Phaeophyceae | Chordaria flagelliformis |
| Fucus evanescens | ||
| Fucus vesiculosus | ||
| Laminaria sp. | ||
| Petalonia fascia | ||
| Ralfsia fungiformis | ||
| Ralfsia verrucosa | ||
| Scytosiphon lomentaria | ||
| Rhodophyta | Florideophyceae | Hildenbrandia rubra |
| Rhodophyceae | Clathromorphum circumscriptum | |
| Polysiphonia sp. | ||
| Porphyra umbilicalis | ||
| Annelida | Polychaeta | Fabricia stellaris |
| Polydora ciliata | ||
| Spirorbis spirorbis | ||
| Anthropoda | Crustacea | Semibalanus balanoides |
| Cnidaria | Anthozoa | Aulactinia stella |
| Clava multicornis | ||
| Hydrozoa | Obelia sp. | |
| Mollusca | Bivalvia | Mytilus edulis and/or Mytilus trossulus |
3.1. Nutrient Enrichment
3.1.1. Alpha Diversity
| Sources of variation | Taxa richness | Shannon-Wiener diversity index | Pielou evenness index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | p | MS | F | p | MS | F | p | |
| Regime | 2 | 13.291 | 4.789 | * | 6.009 | 3.201 | ns | 0.313 | 1.158 | ns |
| Rock pool (Re) | 12 | 2.727 | 1.049 | ns | 1.855 | 6.222 | ** | 0.273 | 3.478 | * |
| Period | 1 | 221.696 | 27.460 | *** | 8.633 | 21.347 | ** | 0.249 | 1.869 | ns |
| Time (P) | 10 | 7.312 | 3.876 | ** | 0.321 | 1.353 | ns | 0.089 | 2.857 | * |
| Re × P | 2 | 6.113 | 2.309 | ns | 0.435 | 1.357 | ns | 0.077 | 1.023 | ns |
| Re × T(P) | 20 | 1.889 | 1.023 | ns | 0.237 | 1.105 | ns | 0.031 | 0.916 | ns |
| P × R(Re) | 12 | 2.599 | 1.415 | ns | 0.298 | 1.389 | ns | 0.079 | 2.318 | * |
| T(P) × R(Re) | 120 | 1.837 | 1.479 | ** | 0.215 | 1.087 | ns | 0.034 | 1.450 | ** |
| Residual | 360 | 1.243 | 0.197 | 0.023 | ||||||
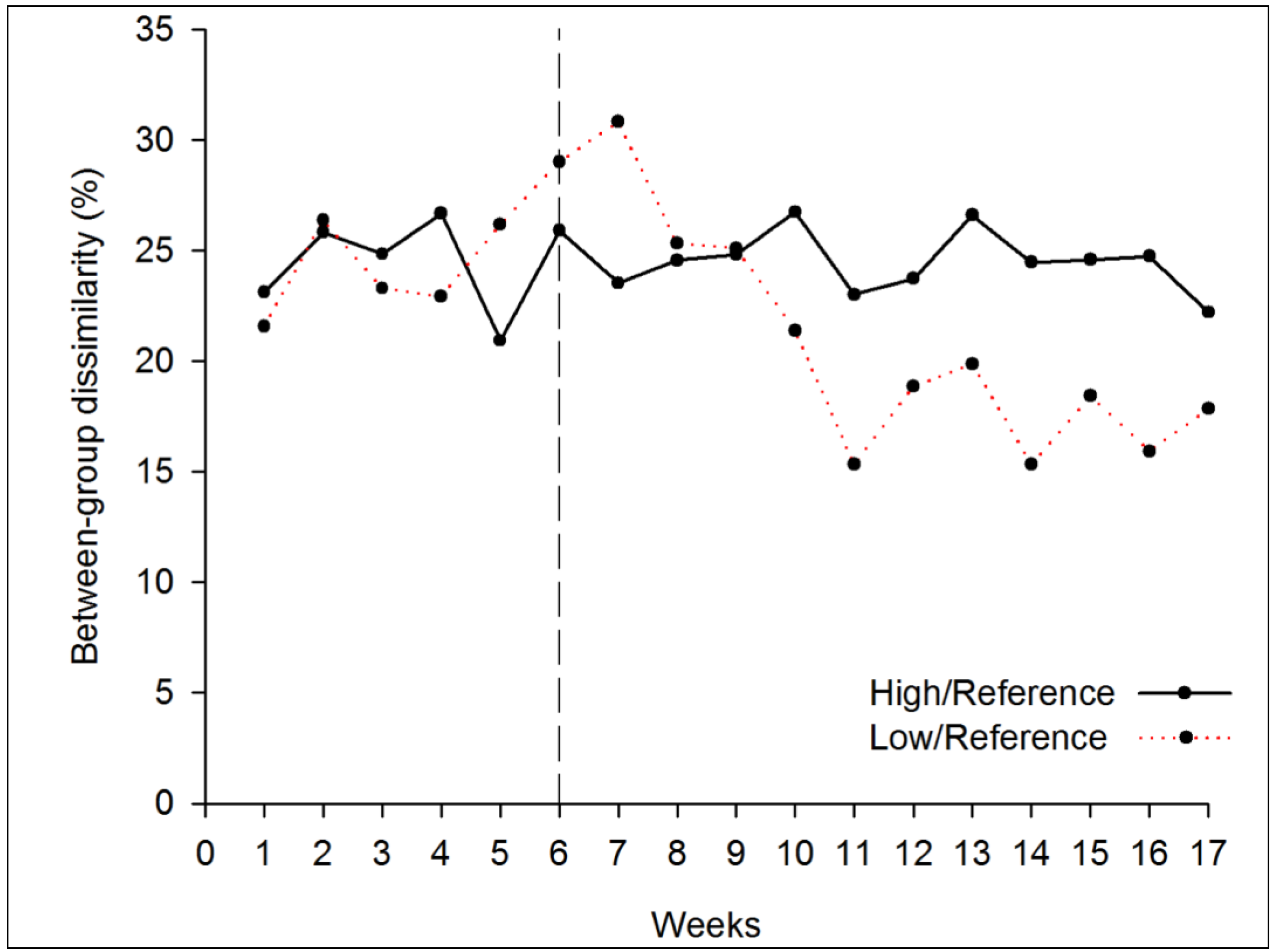
3.1.2. Beta Diversity
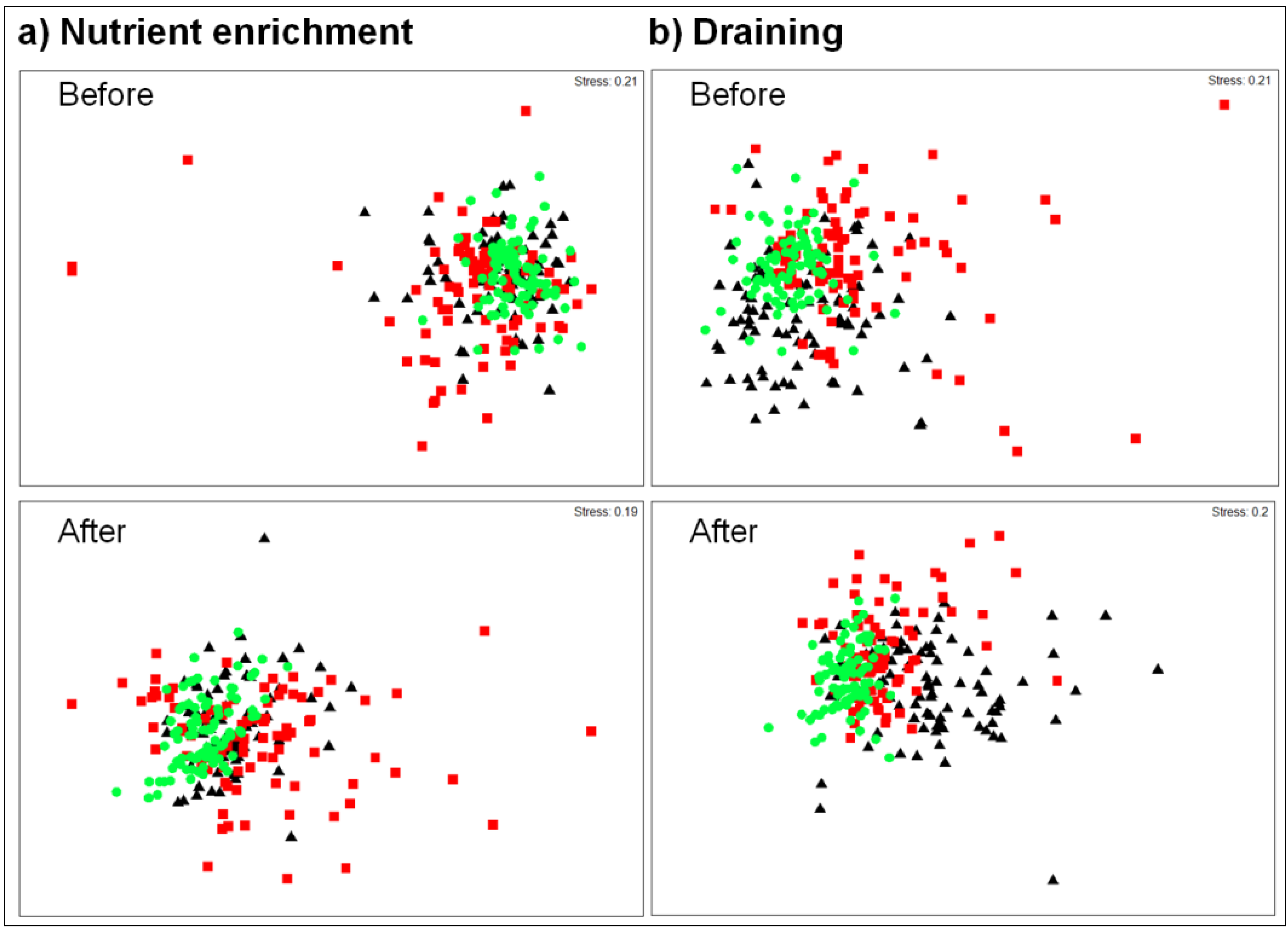
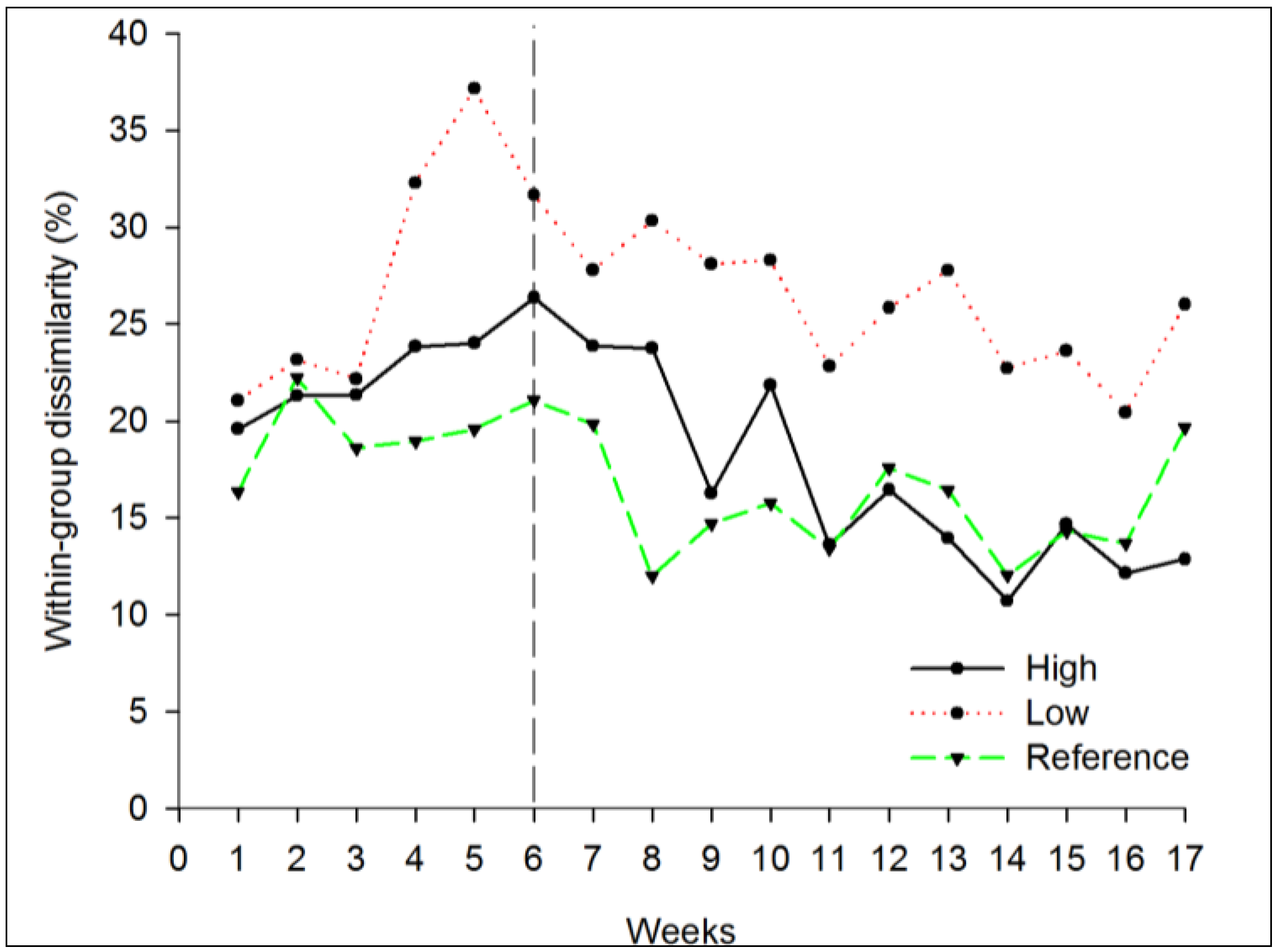
3.2. Draining
3.2.1. Alpha Diversity
| Taxa richness | Shannon-Wiener diversity index | Pielou evenness index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | df | MS | F | p | MS | F | p | MS | F | p |
| Regime | 2 | 65.272 | 11.922 | *** | 5.277 | 12.116 | *** | 0.363 | 1.731 | ns |
| Rock pool (Re) | 12 | 4.316 | 1.015 | ns | 0.406 | 2.495 | ns | 0.211 | 3.958 | * |
| Period | 1 | 69.696 | 5.868 | * | 0.238 | 0.710 | ns | 0.057 | 0.799 | ns |
| Time (P) | 10 | 9.497 | 3.134 | * | 0.214 | 2.987 | * | 0.042 | 1.888 | ns |
| Re × P | 2 | 35.091 | 6.486 | ** | 0.054 | 0.279 | ns | 0.125 | 2.388 | ns |
| Re × T(P) | 20 | 3.030 | 1.620 | ns | 0.072 | 1.698 | * | 0.022 | 0.960 | ns |
| P × R(Re) | 12 | 4.251 | 2.272 | * | 0.163 | 3.861 | *** | 0.053 | 2.326 | ** |
| T(P) × R(Re) | 120 | 1.871 | 1.169 | ns | 0.042 | 0.510 | ns | 0.023 | 1.007 | ns |
| Residual | 360 | 1.600 | 0.083 | 0.022 | ||||||
3.2.2. Beta Diversity
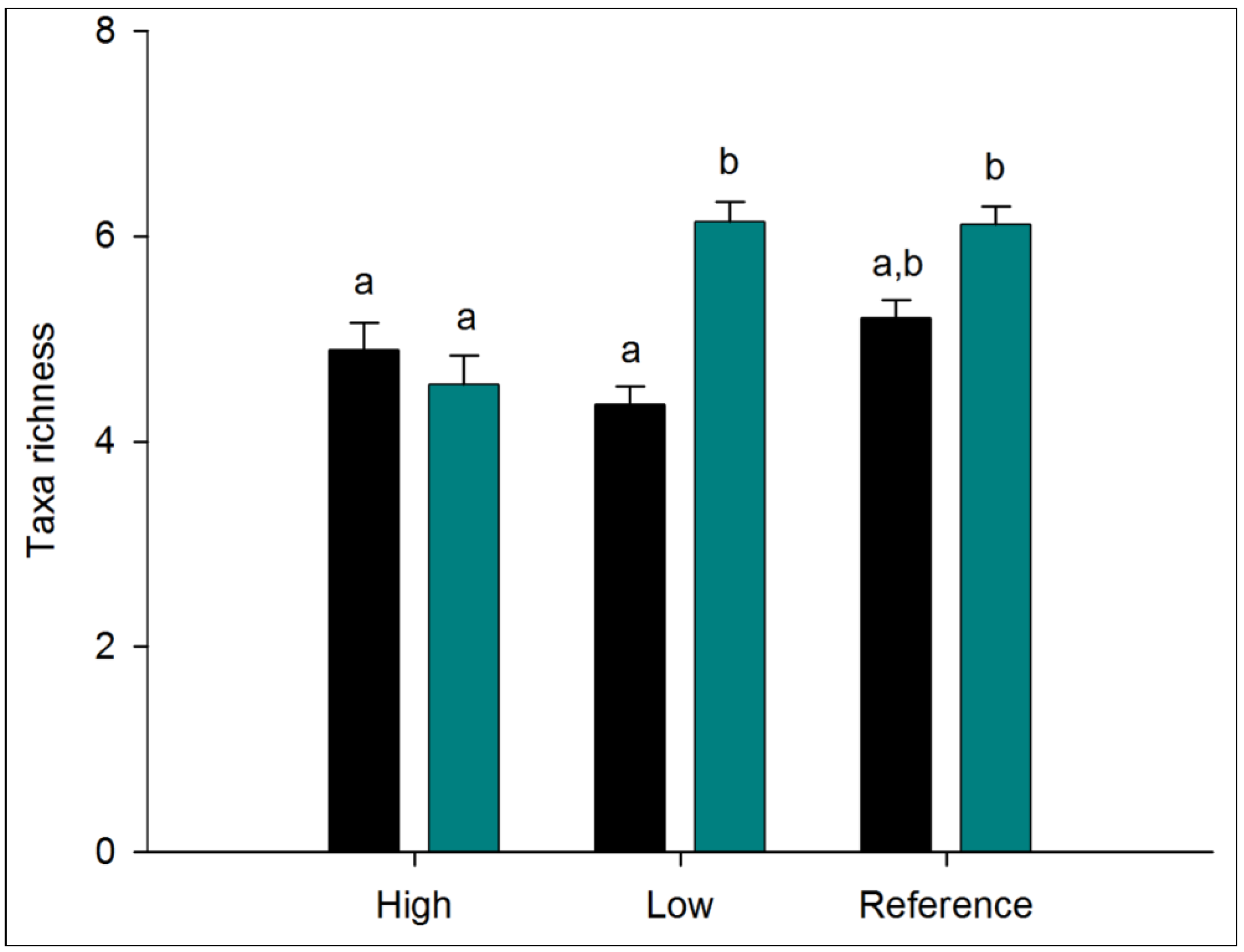
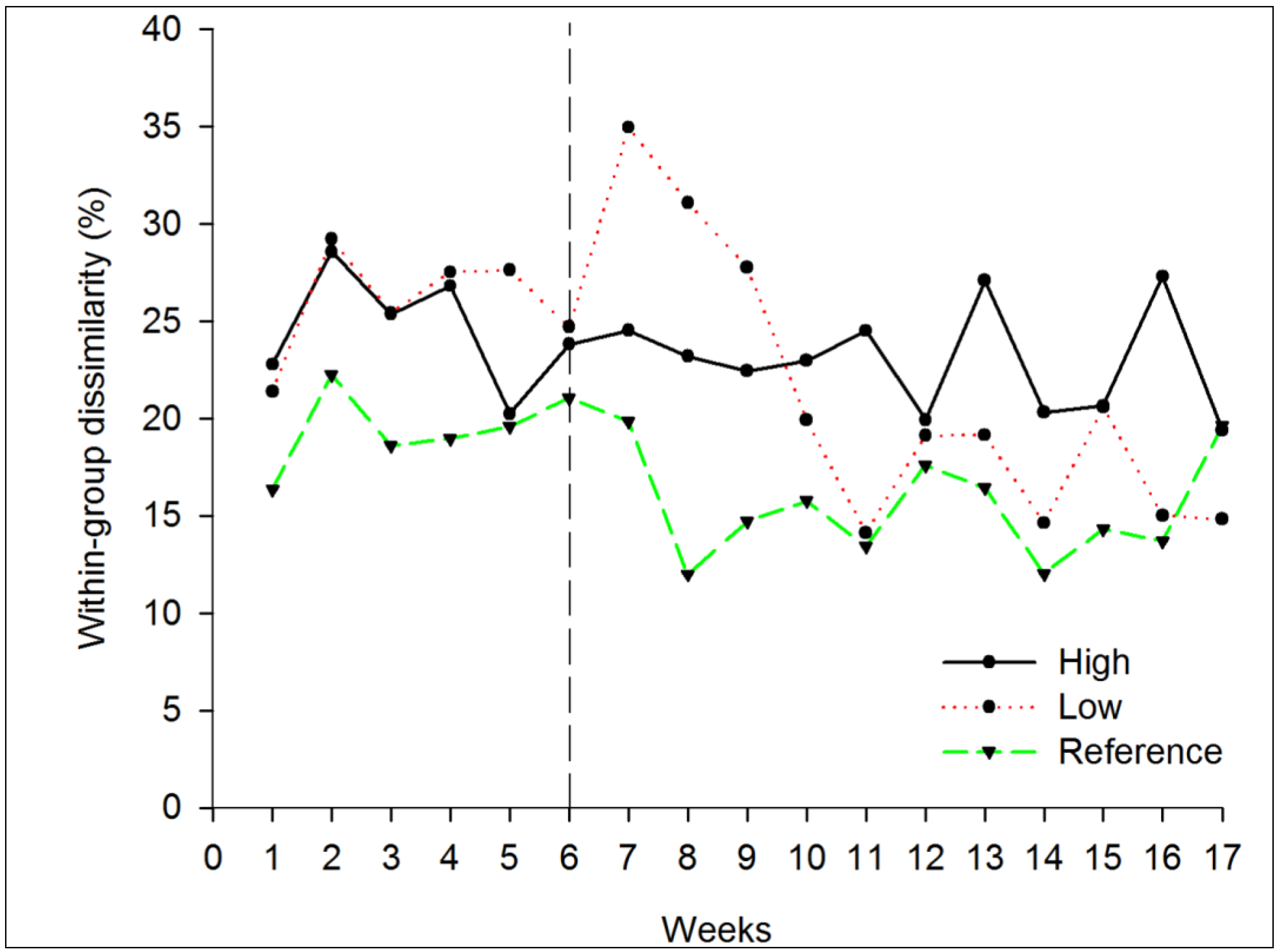
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sousa, W.P. The role of disturbance in natural communities. Annu. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar]
- White, P.S.; Pickett, S.T.A. Natural Disturbance and Patch Dynamics: An Introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic press: Orlando, FL, USA, 1985; pp. 3–13. [Google Scholar]
- Chase, J.M. Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef]
- Gravel, D.; Guichard, F.; Hochberg, M.E. Species coexistence in a variable world. Ecol. Lett. 2011, 14, 828–839. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Cadotte, M.W. Competition-colonization trade-offs and disturbance effects at multiple scales. Ecology 2007, 88, 823–829. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Underwood, A.J. On Beyond BACI: Sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 1994, 4, 3–15. [Google Scholar] [CrossRef]
- Underwood, A.J. Beyond BACI: the detection of environmental impacts on populations in the real, but variable, world. J. Exp. Mar. Biol. Ecol. 1992, 161, 145–178. [Google Scholar] [CrossRef]
- Underwood, A.J. Beyond BACI: Experimental-designs for detecting human environmental impacts on temporal variations in natural-populations. Aust. J. Mar. Fresh. Res. 1991, 42, 569–587. [Google Scholar] [CrossRef]
- Bertocci, I.; Araújo, R.; Vaselli, S.; Sousa-Pinto, I. Marginal populations under pressure: spatial and temporal heterogeneity of Ascophyllum nodosum and associated assemblages affected by human trampling in Portugal. Mar. Ecol. Prog. Ser. 2011, 439, 73–82. [Google Scholar]
- Krasnov, B.R.; Mouillot, D.; Shenbrot, G.I.; Khokhlova, I.S.; Poulin, R. Beta-specificity: The turnover of host species in space and another way to measure host specificity. Int. J. Parasitol. 2011, 41, 33–41. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L. The importance of the variance around the mean effect size of ecological processes. Ecology 2003, 84, 2335–2346. [Google Scholar] [CrossRef]
- Lindegarth, M.; Valentinsson, D.; Hansson, M.; Ulmestrand, M. Effects of trawling disturbances on temporal and spatial structure of benthic soft-sediment assemblages in Gullmarsfjorden, Sweden. ICES J. Mar. Sci. 2000, 57, 1369–1376. [Google Scholar] [CrossRef]
- Fraterrigo, J.M.; Rusak, J.A. Disturbance-driven changes in the variability of ecological patterns and processes. Ecol. Lett. 2008, 11, 756–770. [Google Scholar] [CrossRef]
- Landres, P.B.; Morgan, P.; Swanson, F.J. Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl. 1999, 9, 1179–1188. [Google Scholar]
- Ellingsen, K.; Gray, J.S. Spatial patterns of benthic diversity: is there a latitudinal gradient along the Norwegian continental shelf? J. Anim. Ecol. 2002, 71, 373–389. [Google Scholar] [CrossRef]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Increased variability as a symptom of stress in marine communities. J. Exp. Mar. Bio. Ecol. 1993, 172, 215–226. [Google Scholar] [CrossRef]
- Chapman, M.G.; Underwood, A.J.; Skilleter, G.A. Variability at different spatial scales between a subtidal assemblage exposed to the discharge of sewage and two control assemblages. J. Exp. Mar. Biol. Ecol. 1995, 189, 103–122. [Google Scholar] [CrossRef]
- Caswell, H.; Cohen, J.E. Communities in Patchy Environments: A Model of Disturbance, Competition, and Heterogeneity. In Ecological Heterogeneity; Kolasa, J., Pickett, S.T.A., Eds.; Springer-Verlag: New York, NY, USA, 1991; pp. 97–122. [Google Scholar]
- Cervin, G.; Lindegarth, M.; Viejo, R.M.; Aberg, P. Effects of small-scale disturbances of canopy and grazing on intertidal assemblages on the Swedish west coast. J. Exp. Mar. Biol. Ecol. 2004, 302, 35–49. [Google Scholar] [CrossRef]
- Terlizzi, A.; Benedetti-Cecchi, L.; Bevilacqua, S.; Fraschetti, S.; Guidetti, P.; Anderson, M.J. Multivariate and univariate asymmetrical analyses in environmental impact assessment: a case study of Mediterranean subtidal sessile assemblages. Mar. Ecol. Prog. Ser. 2005, 289, 27–42. [Google Scholar] [CrossRef]
- Dye, A.H. Persistent effects of physical disturbance on meiobenthos in mangrove sediments. Mar. Environ. Res. 2006, 62, 341–355. [Google Scholar] [CrossRef]
- Garpe, K.C.; Yahya, S.A.S.; Lindahl, U.; Ohman, M.C. Long-term effects of the 1998 coral bleaching event on reef fish assemblages. Mar. Ecol. Prog. Ser. 2006, 315, 237–247. [Google Scholar] [CrossRef]
- Wildsmith, M.; Rose, T.; Potter, I.; Warwick, R.; Clarke, K.; Valesini, F.J. Changes in the benthic macroinvertebrate fauna of a large microtidal estuary following extreme modifications aimed at reducing eutrophication. Mar. Pollut. Bull. 2009, 58, 1250–1262. [Google Scholar] [CrossRef]
- Archambault, P.; Banwell, K.; Underwood, A.J. Temporal variation in the structure of intertidal assemblages following the removal of sewage. Mar. Ecol. Prog. Ser. 2001, 222, 51–62. [Google Scholar] [CrossRef]
- Fraschetti, S.; Bianchi, C.N.; Terlizzi, A.; Fanelli, G.; Morri, C.; Boero, F. Spatial variability and human disturbance in shallow subtidal hard substrate assemblages: a regional approach. Mar. Ecol. Prog. Ser. 2001, 212, 1–12. [Google Scholar] [CrossRef]
- Bishop, M.J.; Underwood, A.J.; Archambault, P. Sewage and environmental impacts on rocky shores: necessity of identifying relevant spatial scales. Mar. Ecol. Prog. Ser. 2002, 236, 121–128. [Google Scholar] [CrossRef]
- Collins, S.L. Disturbance frequency and community stability in native tallgrass prairie. Am. Nat. 2000, 155, 311–325. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, Ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Tewfik, A.; Guichard, F.; McCann, K.S. Influence of acute and chronic disturbance on macrophyte landscape zonation. Mar. Ecol. Prog. Ser. 2007, 335, 111–121. [Google Scholar] [CrossRef]
- Metaxas, A.; Scheibling, R.E. Community structure and organization of tidepools. Mar. Ecol. Prog. Ser. 1993, 98, 187–198. [Google Scholar] [CrossRef]
- Hunt, H.L.; Scheibling, R.E. Structure and dynamics of mussel patches in tidepools on a rocky shore in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 1995, 124, 105–115. [Google Scholar] [CrossRef]
- Underwood, A.J.; Skilleter, G.A. Effects of patch-size on the structure of assemblages in rock pools. J. Exp. Mar. Biol. Ecol. 1996, 197, 63–90. [Google Scholar] [CrossRef]
- O’Connor, N.E.; Crowe, T.P. Biodiversity loss and ecosystem functioning: Distinguishing between number and identity of species. Ecology 2005, 86, 1783–1796. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Cinelli, F. Patterns of disturbance and recovery in littoral rock pools: Nonhierarchical competition and spatial variability in secondary succession. Mar. Ecol. Prog. Ser. 1996, 135, 145–161. [Google Scholar] [CrossRef]
- Bergeron, P.; Bourget, E. Shore topography and spatial partitioning of crevice refuges by sessile epibenthos in an ice disturbed environment. Mar. Ecol. Prog. Ser. 1986, 28, 129–145. [Google Scholar] [CrossRef]
- Archambault, P.; Bourget, E. Influence of shoreline configuration on spatial variation of meroplanktonic larvae, recruitment and diversity of benthic subtidal communities. J. Exp. Mar. Biol. Ecol. 1999, 238, 161–184. [Google Scholar] [CrossRef]
- Smith, G.K.; Guichard, F.; Petrovic, F.; McKindsey, C.W. Using spatial statistics to infer scales of demographic connectivity between populations of the blue mussel, Mytilus spp. Limnol. Oceanogr. 2009, 54, 970–977. [Google Scholar] [CrossRef]
- Poulin, P. Cycle biogéochimique de l’azote dans l’estuaire du Saint-Laurent; rôle des marais côtiers. Ph.D. Thesis, Université du Québec à Rimouski, Rimouski, Canada, 2008. [Google Scholar]
- Aminot, A.; Kérouel, R. Hydrologie des écosystèmes marins: paramètres et analyses; (in French). Ifremer: Plouzané, France, 2004; pp. 215–282. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; pp. 87–104. [Google Scholar]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a biological effects study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Gaston, K.J.; Mcardle, B.H. The temporal variability of animal abundances: Measures, Methods and patterns. Philos T. Roy. Soc. B. 1994, 345, 335–358. [Google Scholar] [CrossRef]
- Archambault, P.; Bourget, E. Scales of coastal heterogeneity and benthic intertidal species richness, Diversity and abundance. Mar. Ecol. Prog. Ser. 1996, 136, 111–121. [Google Scholar] [CrossRef]
- Apitz, S.E.; Degetto, S.; Cantaluppi, C. The use of statistical methods to separate natural background and anthropogenic concentrations of trace elements in radio-chronologically selected surface sediments of the Venice Lagoon. Mar. Pollut. Bull. 2009, 58, 402–414. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Cinelli, F.; Benedetti-Cecchi, L. Patterns of spatial variability in epiphytes of Posidonia oceanica - Differences between a disturbed and two reference locations. Aquat. Bot. 2004, 79, 345–356. [Google Scholar] [CrossRef]
- Münkemüller, T.; de Bello, F.; Meynard, C.; Gravel, D.; Lavergne, S.; Mouillot, D.; Mouquet, N.; Thuiller, W. From diversity indices to community assembly processes: A test with simulated data. Ecography 2012, 35, 468–480. [Google Scholar] [CrossRef]
- Drouin, A.; Sirois, P.; Archambault, P. Discriminating zooplankton communities in lakes with brook trout (Salvelinus. fontinalis) and in fishless lakes. Ecoscience 2009, 16, 271–281. [Google Scholar] [CrossRef]
- Terlizzi, A.; Fraschetti, S.; Guidetti, P.; Boero, F. The effects of sewage discharge on shallow hard substrate sessile assemblages. Mar. Pollut. Bull. 2002, 44, 544–550. [Google Scholar] [CrossRef]
- Hillebrand, H.; Sommer, U. Response of epilithic microphytobenthos of the Western Baltic Sea to in situ experiments with nutrient enrichment. Mar. Ecol. Prog. Ser. 1997, 160, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Crowe, T.P.; Thompson, R.C.; Bray, S.; Hawkins, S.J. Impacts of anthropogenic stress on rocky intertidal communities. J. Aquat. Ecosyst. Stress Recov. 2000, 7, 273–297. [Google Scholar] [CrossRef]
- Lepori, F.; Malmqvist, B. Deterministic control on community assembly peaks at intermediate levels of disturbance. Oikos 2009, 118, 471–479. [Google Scholar] [CrossRef]
- Petraitis, P.S.; Methratta, E.T. Using patterns of variability, to test for multiple community states on rocky intertidal shores. J. Exp. Mar. Biol. Ecol. 2006, 338, 222–232. [Google Scholar] [CrossRef]
- Dakos, V.; van Nes, E.H.; D’Odorico, P.; Scheffer, M. Robustness of variance and autocorrelation as indicators of critical slowing down. Ecology 2012, 93, 264–271. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Séguin, A.; Gravel, D.; Archambault, P. Effect of Disturbance Regime on Alpha and Beta Diversity of Rock Pools. Diversity 2014, 6, 1-17. https://doi.org/10.3390/d6010001
Séguin A, Gravel D, Archambault P. Effect of Disturbance Regime on Alpha and Beta Diversity of Rock Pools. Diversity. 2014; 6(1):1-17. https://doi.org/10.3390/d6010001
Chicago/Turabian StyleSéguin, Annie, Dominique Gravel, and Philippe Archambault. 2014. "Effect of Disturbance Regime on Alpha and Beta Diversity of Rock Pools" Diversity 6, no. 1: 1-17. https://doi.org/10.3390/d6010001




