Seasonal Changes in Microbial Community Structure in Freshwater Stream Sediment in a North Carolina River Basin
Abstract
:1. Introduction
2. Methods
2.1. Study Area and Site Descriptions
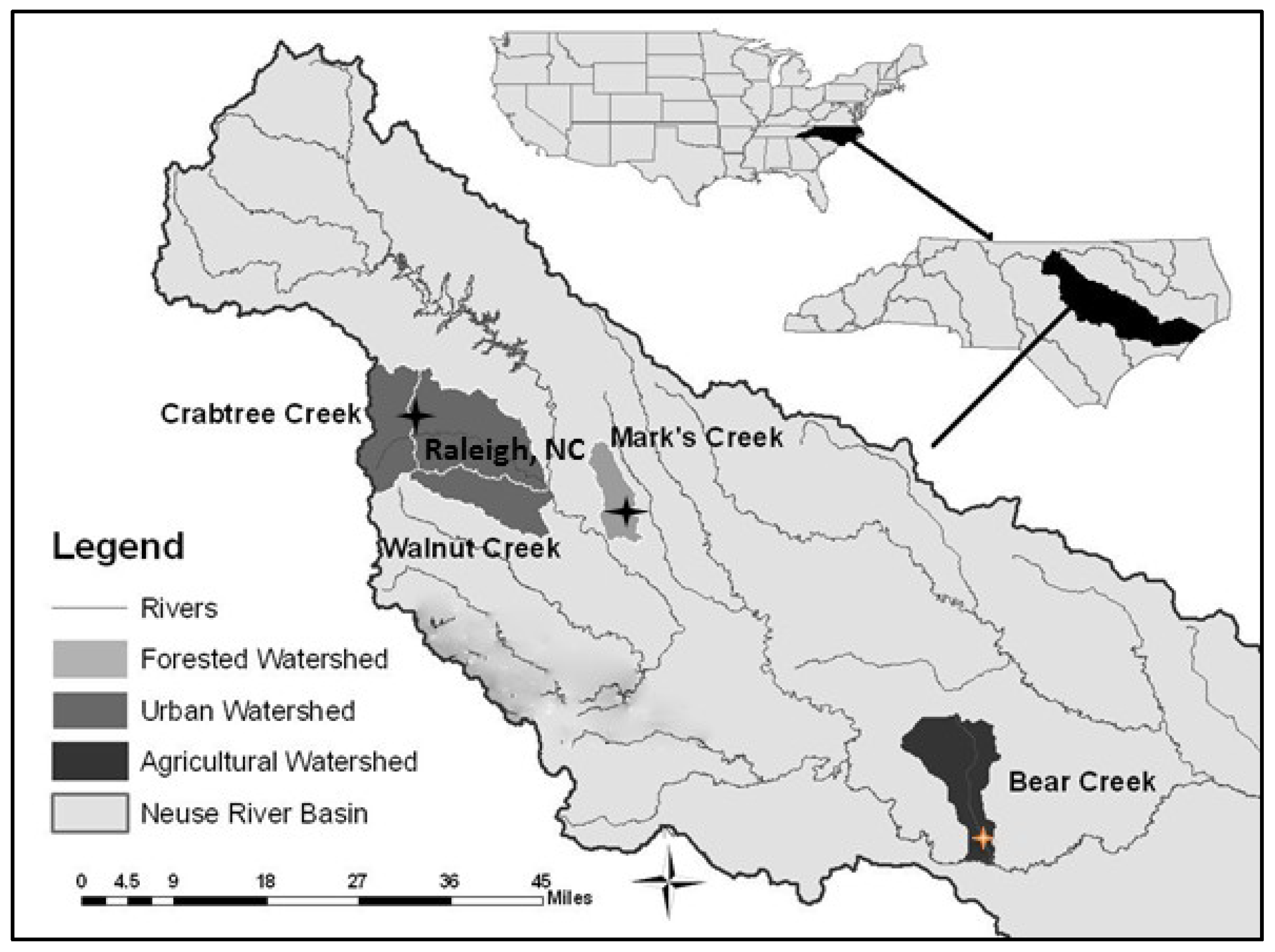
2.2. Sediment Microbial Sampling
| Samples | Collection Date | Mean Daily Discharge (cfs) * (10 year median) | Mean Daily Air Temp * (°F) | Average Daily Precipitation (Inches) | SWI | Evenness |
|---|---|---|---|---|---|---|
| Summer Season | ||||||
| MD-07c | 15 September 2007 | 10%–20% | 72 | 0.15 | 4.2 | 0.85 |
| MD-07f | 15 September 2007 | 10%–20% | 72 | 0.15 | 4.0 | 0.87 |
| MD-08c | 20 June 2008 | 198 (223) | 78 | 0.12 | 4.4 | 0.87 |
| MD-08f | 20 June 2008 | 198 (223) | 78 | 0.12 | 4.2 | 0.86 |
| BD-07f | 15 September 2007 | 10%–20% | 72 | 0.15 | 4.0 | 0.91 |
| BD-07f2 | 15 September 2007 | 10%–20% | 72 | 0.15 | 3.7 | 0.89 |
| BD-08c | 25 June 2008 | 14 (24) | 79 | 0.12 | 3.6 | 0.85 |
| BD-08f | 25 June 2008 | 14 (24) | 79 | 0.12 | 3.9 | 0.85 |
| CD-07f | 10 July 2007 | 2.5 (2.2) | 80 | 0.15 | 4.1 | - |
| CD-08c | 11 June 2008 | 2.8 (2.8) | 76 | 0.11 | 4.0 | 0.84 |
| Fall Season | ||||||
| MW-08c | 21 November 2008 | ** | 51 | 0.11 | 4.0 | 0.91 |
| MW-08f | 19 December 2008 | ** | 42 | 0.09 | 4.3 | 0.91 |
| BW-08c | 3 December 2008 | 74 (40) | 47 | 0.11 | 3.7 | 0.92 |
| BW-08f | 1 November 2008 | 29 (33) | 56 | 0.10 | 3.7 | 0.88 |
| CW-08c | 17 November 2008 | 3.1 (2.8) | 52 | 0.10 | 3.5 | 0.88 |
| CW-08f | 19 December 2008 | 2.9 (2.9) | 42 | 0.09 | 4.1 | 0.92 |
2.3. Stable Isotope Analysis
2.4. Statistical Analyses
2.5. Microbial Analyses—DNA Extraction and Quantification
2.6. PCR Amplification of 16S rDNA
2.7. Restriction Enzyme-Digest of PCR Products
2.8. Analysis of T-RFLP Data
3. Results
3.1. Microbial Structure
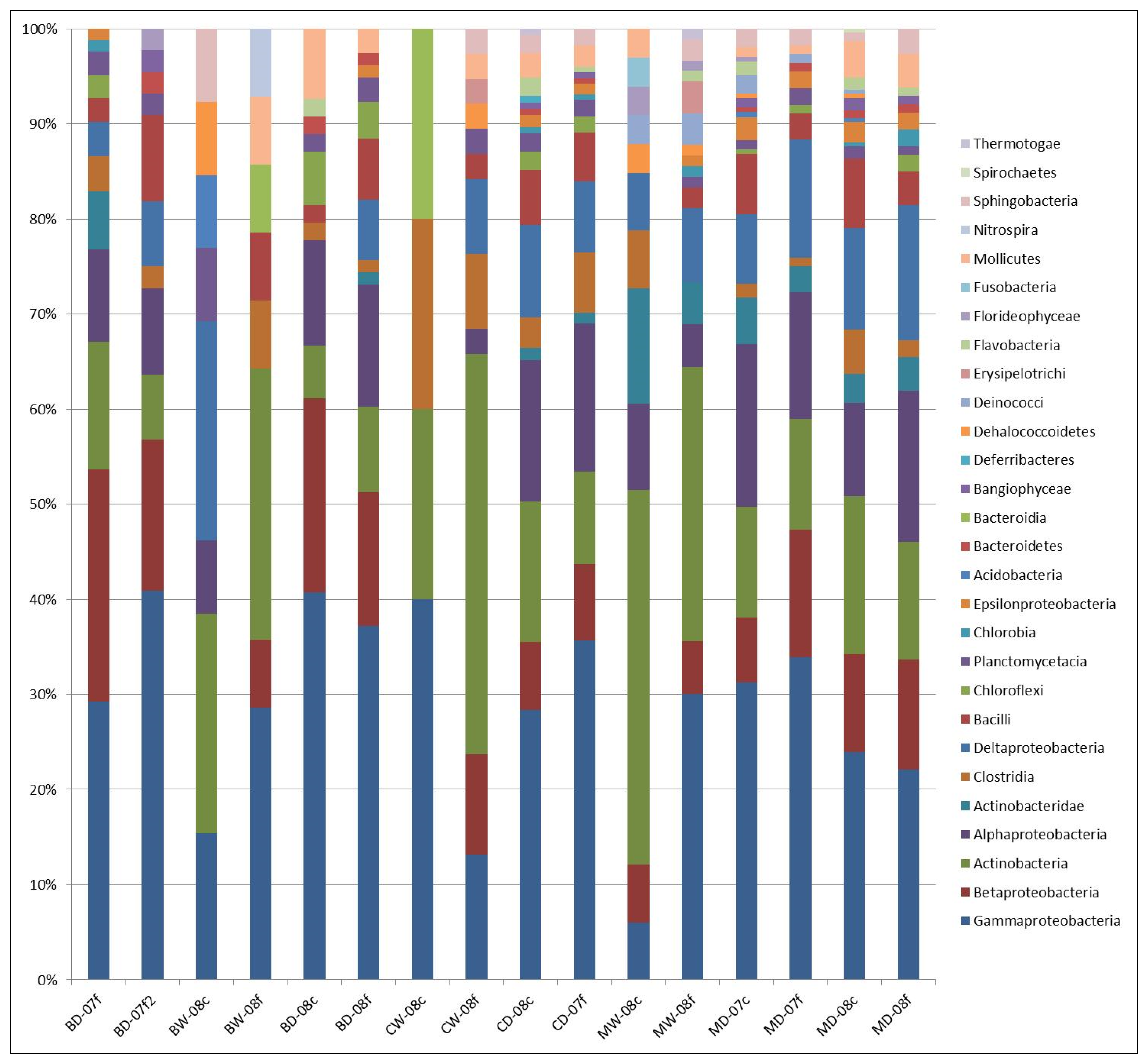
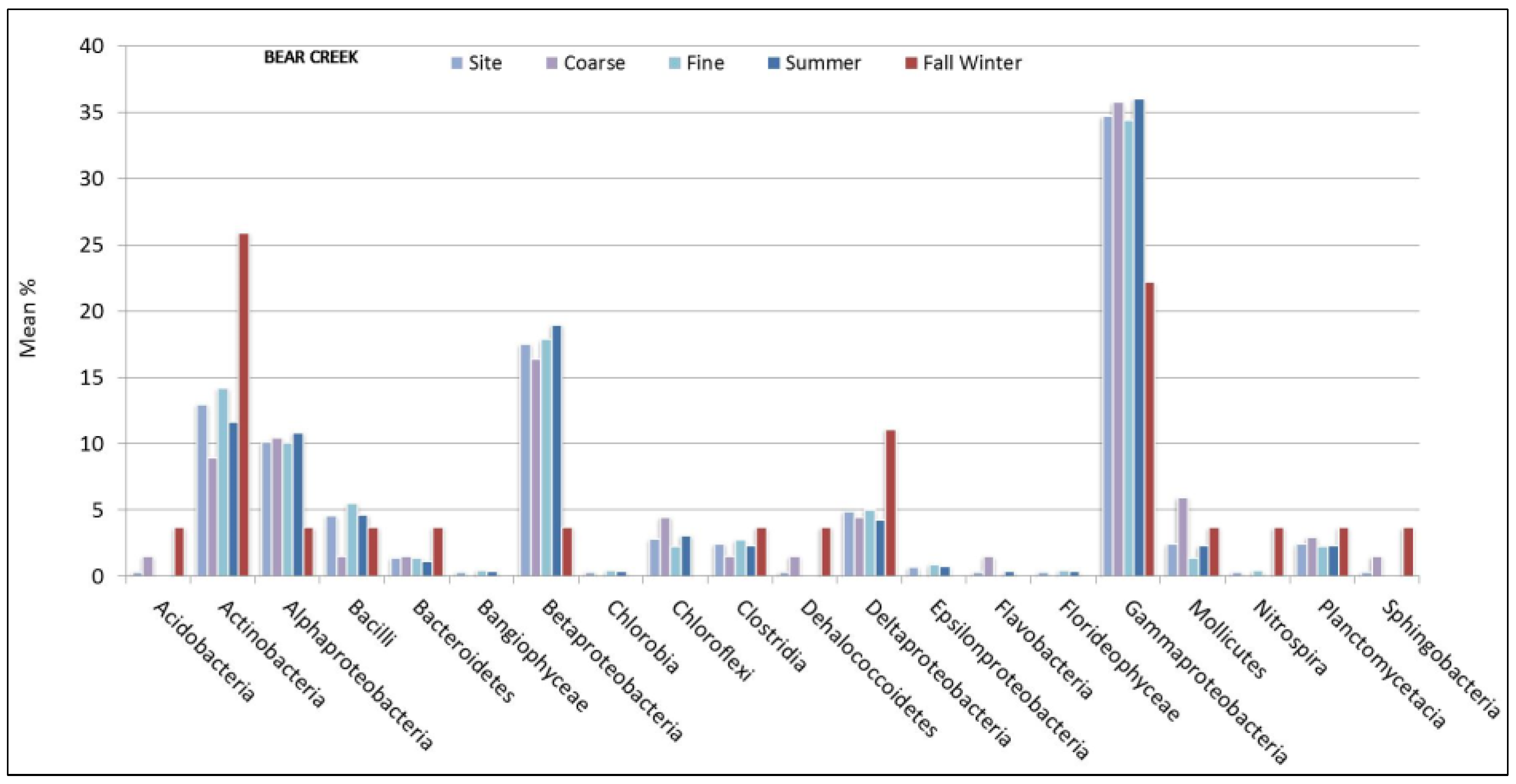
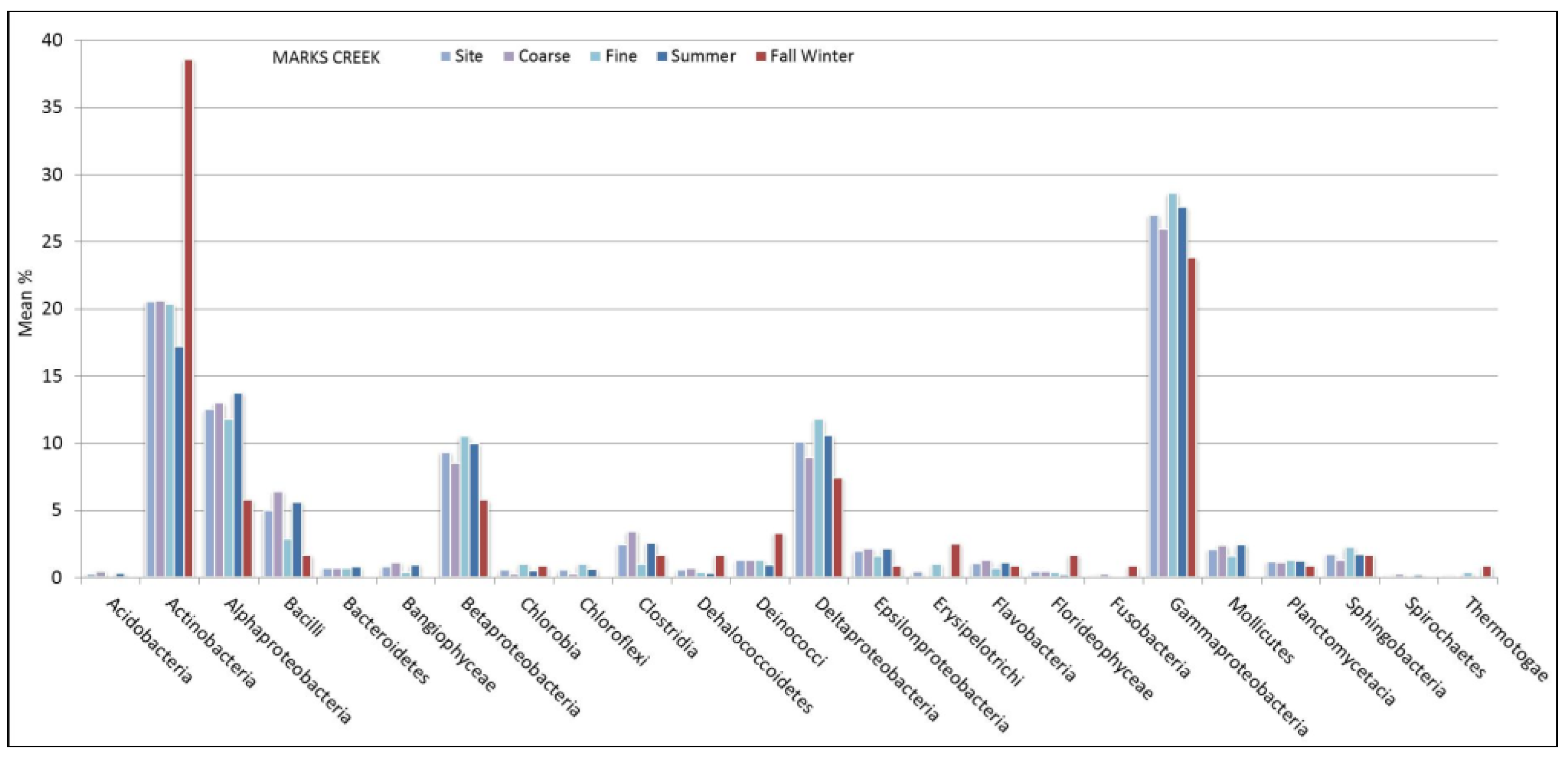

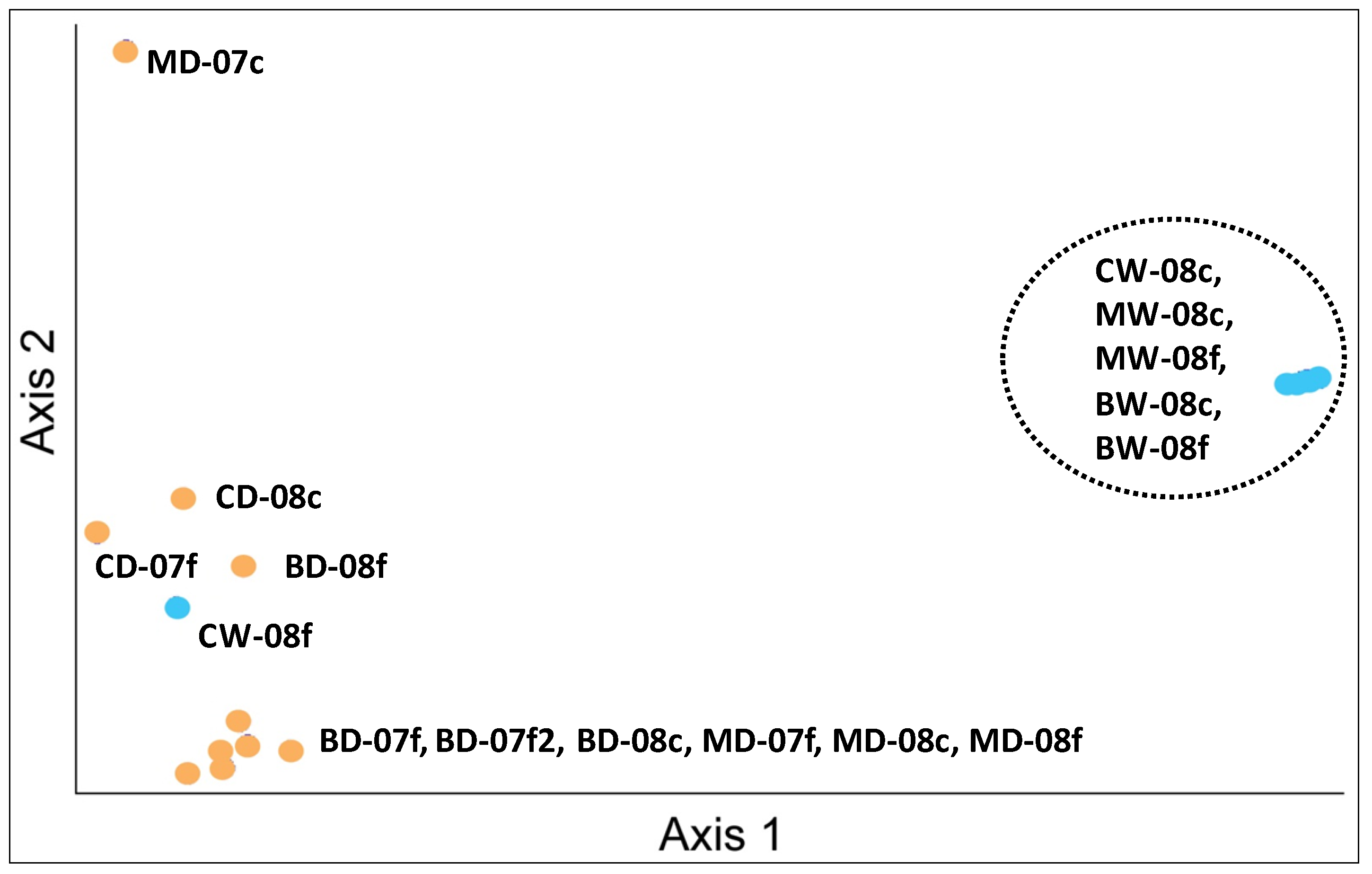
3.2. Sediment Stable Isotopes
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Palmer, M.A.; Covich, A.P.; Lake, S.; Biro, P.; Brooks, J.J.; Cole, J.; Dahm, C.; Gibert, J.; Goedkoop, W.; Martens, K.; et al. Sediment biota and life above sediments as potential drivers of biodiversity and ecological processes. BioScience 2000, 50, 1062–1075. [Google Scholar] [CrossRef]
- Hullar, M.A.; Kaplan, L.A.; Stahl, A. Recurring seasonal dynamics of microbial communities in stream habitats. Appl. Environ. Microbiol. 2006, 72, 713–722. [Google Scholar] [CrossRef]
- Findlay, S.; Tank, J.; Dye, S.; Valett, H.M.; Mulholland, P.J.; McDowell, W.H.; Johnson, S.L.; Hamilton, S.K.; Edmonds, J.; Dodds, W.K.; et al. A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams. Microb. Ecol. 2002, 43, 55–66. [Google Scholar] [CrossRef]
- Wang, S.; Sudduth, E.; Wallenstein, M.D.; Wright, J.P.; Bernhardt, E.M. Watershed urbanization alters the composition and function of stream bacterial communities. PLoS One 2011, 6, e22972. [Google Scholar]
- Chiaramonte, J.B.; Roberto Mdo, C.; Pagioro, T.A. Seasonal dynamics and community structure of bacterioplankton in upper Paraná River floodplain. Microb. Ecol. 2013, 66, 773–783. [Google Scholar] [CrossRef]
- Findlay, S.; Yeates, J.C.; Hullar, M.; Stahl, D.A.; Kaplan, L.A. Biome-level biogeography of streambed microbiota. Appl. Environ. Microb. 2008, 74, 3014–3021. [Google Scholar] [CrossRef]
- Fazi, S.; Vazquez, E.; Casamayor, E.O.; Amalfitano, S.; Butturini, A. Stream hydrological fragmentation drives bacterioplankton community composition. PLoS One 2013, 8, e64109. [Google Scholar]
- Mummey, D.L.; Stahl, P.D. Spatial and temporal variability of bacterial 16S rDNA-based T-RFLP patterns derived from soil of two Wyoming grassland ecosystems. FEMS Microbiol. Ecol. 2003, 46, 113–120. [Google Scholar] [CrossRef]
- Starry, O.S.; Valett, H.M.; Schreiber, M.E. Nitrification rates in a headwater stream: Influences of seasonal variation in C and N supply. J. North Am. Benthol. Soc. 2005, 24, 753–768. [Google Scholar] [CrossRef]
- Arango, C.P.; Tank, J.L. Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. J. North Am. Benthol. Soc. 2008, 27, 90–107. [Google Scholar] [CrossRef]
- Paerl, H.W.; Pinckney, J.L.; Fear, J.M.; Peierls, B.L. Ecosystem responses to internal and watershed organic matter loading: Consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998, 166, 17–25. [Google Scholar] [CrossRef]
- Mallin, M.A.; Cahoon, L.B. Industrialized animal production—a major source of nutrient and microbial pollution to aquatic ecosystems. Popul. Environ. 2003, 24, 369–385. [Google Scholar] [CrossRef]
- Oczkowski, A.; Nixon, S.; Henry, K.; DiMilla, P.; Pilson, M.; Granger, S.; Buckley, B.; Thornber, C.; McKinney, R.; Chaves, J. Distribution and trophic importance of anthropogenic nitrogen in Narragansett Bay: An assessment using stable isotopes. Estuar. Coasts 2008, 31, 53–69. [Google Scholar] [CrossRef]
- Usry, B.P. Nitrogen Loading in the Neuse River Basin, North Carolina: The Rivernet Monitoring Program. Master Thesis, North Carolina State University, Raleigh, NC, USA, May 2005; pp. 90–120. [Google Scholar]
- North Carolina Department of Environmental and Natural Resources (NCDENR). Basin-wide Assessment Reports for the Neuse River Basin, NCDENR: Raleigh, NC, USA, 2009.
- State Climate Office of North Carolina. CRONOS Data Source Map and Archive; North Carolina State University: Raleigh, NC, USA, 2003. Available online: http://www.nc-climate.ncsu.edu/cronos/index.php (accessed on 27 October 2013).
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Fry, B.; Allen, Y.C. Stable isotopes in zebra mussels as bioindicators of river-watershed linkages. River Res. Appl. 2003, 19, 683–696. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Gau, C.; Zhu, J.G.; Zhu, J.Y.; Gao, X.; Dou, Y.J.; Hosen, Y. Nitrogen export from an agriculture watershed in the Taihu Lake area, China. Environ. Geochem. Health 2004, 26, 199–207. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Process. 2001, 15, 1301–1346. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar]
- Strassburger, K.; Bretz, F. Compatible simultaneous lower confidence bounds for the Holm procedure and other Bonferroni-based closed tests. Stat. Med. 2008, 27, 4914–4927. [Google Scholar] [CrossRef]
- Liu, W.T.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar]
- Kent, A.D.; Smith, D.J.; Benson, B.J.; Triplett, E.W. Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl. Environ. Microbiol. 2003, 69, 6768–6776. [Google Scholar] [CrossRef]
- Johnson, M.C.; Devine, A.A.; Ellis, J.C.; Grunden, A.M.; Fellner, V. Effects of antibiotics and oil on microbial profiles and fermentation in mixed cultures of ruminal microorganisms. J. Dairy Sci. 2009, 92, 4467–4480. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Ogata, K.; Nakamura, M.; Matsui, H.; Benno, Y. Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol. Ecol. 1999, 29, 159–169. [Google Scholar] [CrossRef]
- Blackwood, C.B.; Hudleston, D.; Zak, D.R.; Buyer, J.S. Interpreting ecological diversity indices applied to terminal restriction length polymorphism data: Insights from simulated microbial communities. Appl. Environ. Microbiol. 2007, 73, 5276–5283. [Google Scholar] [CrossRef]
- Hewson, I.; Jacobson Meyers, M.F.; Fuhrman, J.A. Diversity and biogeography of bacterial assemblages in surface sediments across the San Pedro Basin, Southern California Borderlands. Environ. Microbiol. 2007, 9, 923–933. [Google Scholar] [CrossRef]
- Crump, B.; Hobbie, J.E. Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol. Oceanogr. 2005, 50, 1718–1729. [Google Scholar] [CrossRef]
- Judd, K.E.; Crump, B.C.; Kling, G.W. Variation in dissolved organic matter controls bacterial production and community composition. Ecology 2006, 87, 2068–2079. [Google Scholar] [CrossRef]
- Sezenna, M.L. (Ed.) Proteobacteria: Phylogeny, Metabolic Diversity and Ecological Effects; Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2011.
- Adams, H.E.; Crump, B.C.; Kling, G.W. Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environ. Microbiol. 2010, 12, 1319–1333. [Google Scholar] [CrossRef]
- Nelson, J.D.; Boehme, S.E.; Reimers, C.E. Temporal patterns of microbial community structure in the Mid-Atlantic Bight. FEMS Microbiol. Ecol. 2008, 65, 484–493. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Rees, G.N.; Watson, G.; Williams, J. The potential of sediment microbial community structure as either a target or an indicator for the restoration of degraded aquatic ecosystems—preliminary results from a creek affected by in-stream sand accretion. Ecol. Manag. Restor. 2008, 9, 156–159. [Google Scholar] [CrossRef]
- Kan, J.; Suzuki, M.T.; Wang, K.; Evans, S.E.; Chen, F. High temporal but low spatial heterogeneity of bacterioplankton in the Chesapeake Bay. Appl. Environ. Microbiol. 2007, 73, 6776–6789. [Google Scholar] [CrossRef]
- Boggs, J.L.; Sun, G.; McNulty, S.G.; Swartley, W.; Treasure, E.; Summer, W. Temporal and Spatial Variability in North Carolina Piedmont Stream Temperature. In Proceedings of the 2009 AWRA Spring Specialty Conference, Anchorage, AK, USA, 4–6 May 2009.
- Portillo, M.C.; Anderson, S.P.; Fierer, N. Temporal variability in the diversity and composition of stream bacterioplankton communities. Environ. Microbiol. 2012, 14, 2417–2428. [Google Scholar] [CrossRef]
- Rees, G.N.; Watson, G.O.; Baldwin, D.S.; Mitchell, A.M. Variability in sediment microbial communities in a semipermanent stream: Impact of drought. J. North Am. Benthol. Soc. 2006, 25, 370–378. [Google Scholar] [CrossRef]
- Lenat, D.R.; Crawford, J.K. Effects of land use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia 1994, 294, 185–199. [Google Scholar] [CrossRef]
- Galloway, J.N. Nitrogen cycles past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Cebron, A.; Garnier, J. Nitrobacter and Nitrospira genera as representatives of nitrite-oxidizing bacteria: Detection, quantification and growth along the lower Seine River (France). Water Res. 2005, 39, 4979–4992. [Google Scholar] [CrossRef]
- Rubin, M.A.; Leff, L.G. Nutrients and other abiotic factors affecting bacterial communities in an Ohio River (USA). Microbiol. Ecol. 2007, 54, 374–383. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Robertson, G.P. The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology 2000, 81, 1402–1414. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Colloff, M.J.; Kookana, R.S. Effect of wastewater treatment plant effluent on microbial function and community structure in the sediment of a freshwater stream with variable seasonal flow. Appl. Environ. Microbiol. 2008, 74, 2659–2668. [Google Scholar] [CrossRef]
- Wagner, M.; Rath, G.; Amann, R.; Koops, H.P.; Schleifer, K.H. In situ, identification of ammonia-oxidizing bacteria. Syst. Appl. Microbiol. 1995, 18, 251–264. [Google Scholar] [CrossRef]
- Karr, J.D.; Showers, W.J.; Gilliam, J.W.; Andres, A.S. Tracing nitrate transport and environmental impact from intensive swine farming using delta Nitrogen-15. J. Environ. Qual. 2001, 30, 1163–1175. [Google Scholar] [CrossRef]
- Line, D.E.; White, N.M.; Osmond, D.L.; Jennings, G.D.; Mojonnier, C.B. Pollutant export from various land uses in the upper Neuse River Basin. Water Environ. Res. 2002, 74, 100–108. [Google Scholar] [CrossRef]
- Gustafson, L.L.; Showers, W.J.; Thomas, K.; Levine, J.F.; Stoskopf, M. Temporal and spatial variability in stable isotope compositions of a freshwater mussel: Implications for biomonitoring and ecological studies. Oecologia 2007, 152, 140–150. [Google Scholar]
- Bucci, J.P.; Showers, W.J.; Genna, B.; Levine, J.F. Stable oxygen and carbon isotope profiles in an invasive bivalve (Corbicula fluminea) in North Carolina watersheds. Geochim. Cosmochim. Acta 2009, 73, 3234–3247. [Google Scholar] [CrossRef]
- Kendall, C. Tracing Nitrogen Sources and Cycling in Catchments. In Isotope Tracers in Catchment Hydrology; Kendall, C., McDonnell, J.J., Eds.; Elsevier: New York, NY, USA, 1998; pp. 519–576. [Google Scholar]
- Barnes, R.T.; Raymond, P.A. Land use controls on the delivery, processing, and removal of nitrogen from small watersheds: Insights from the dual isotopic composition of stream nitrate. Ecol. Appl. 2010, 20, 1961–1978. [Google Scholar] [CrossRef]
- Howarth, R.W.; Boyer, E.W.; Pabich, W.; Galloway, J.N. Nitrogen use in the United States from 1961 to 2000 and potential future trends. Ambio 2002, 31, 88–96. [Google Scholar]
- McClelland, J.W.; Valiela, I.; Michener, R.H. Nitrogen-stable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds. Limnol. Oceanogr. 1997, 42, 930–937. [Google Scholar] [CrossRef]
- McKinney, R.A.; Lake, J.L.; Charpentier, M.A. Using mussel isotope ratios to assess anthropogenic nitrogen inputs to freshwater ecosystems. Environ. Monit. Assess. 2002, 74, 167–192. [Google Scholar] [CrossRef]
- Savage, C.; Leavitt, P.R.; Elmgren, R. Distribution and retention of effluent nitrogen in surface sediments of a coastal bay. Limnol. Oceanogr. 2004, 49, 1503–1511. [Google Scholar] [CrossRef]
- Fierer, N.; Morse, J.L.; Berthrong, S.T.; Bernhardt, E.S.; Jackson, R.B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 2007, 88, 2162–2173. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bucci, J.P.; Szempruch, A.J.; Caldwell, J.M.; Ellis, J.C.; Levine, J.F. Seasonal Changes in Microbial Community Structure in Freshwater Stream Sediment in a North Carolina River Basin. Diversity 2014, 6, 18-32. https://doi.org/10.3390/d6010018
Bucci JP, Szempruch AJ, Caldwell JM, Ellis JC, Levine JF. Seasonal Changes in Microbial Community Structure in Freshwater Stream Sediment in a North Carolina River Basin. Diversity. 2014; 6(1):18-32. https://doi.org/10.3390/d6010018
Chicago/Turabian StyleBucci, John P., Anthony J. Szempruch, Jane M. Caldwell, Joseph C. Ellis, and Jay F. Levine. 2014. "Seasonal Changes in Microbial Community Structure in Freshwater Stream Sediment in a North Carolina River Basin" Diversity 6, no. 1: 18-32. https://doi.org/10.3390/d6010018




