Contrasting Patterns of Phytoplankton Assemblages in Two Coastal Ecosystems in Relation to Environmental Factors (Corsica, NW Mediterranean Sea)
Abstract
:1. Introduction
| Study sites | References | Study periods | Periods of high biomass | Dominant taxonomic groups |
|---|---|---|---|---|
| Catalan coast (Spain) | [7] | Winter | February/March | - Diatoms (Chaetoceros sp., Detonula pumila) |
| Banyuls-sur-Mer (France) | [30] | Annual cycle | February | - Diatoms (Skeletonema costatum)Cryptophytes; |
| April/May | - Diatoms (Chaetoceros sp., Nitzschia sp., Rhizolenia delicatula) | |||
| Autumn | - Diatoms | |||
| [14] | Annual cycle | March/April | - Diatoms (Chaetoceros sp., Pseudo-nitzschia calliantha, Rhizosolenia sp.), Dinoflagellates (Prorocentrum sp., Protoperidinium sp.); | |
| Summer | - Diatoms (Leptocylindrus sp.), Dinoflagellates (Ceratium sp., Gyrodinium sp., Gymnodinium sp., Heterocapsa sp.), Cyanobacteria (Synechococcus sp., ProChlorococcus sp.) | |||
| Marseilles (France) | [13] | Annual cycle | February | - Diatoms (Skeletonema costatum, Chaetoceros sp., Rhizosolenia stolterfothii); |
| March/May | - Diatoms (Skeletonema costatum, Chaetoceros curvisetus, Lauderia annulata); | |||
| Autumn | - Diatoms (Skeletonema costatum, Leptocylindrus danicus, Thalassionema nitzschioides, Thalassiothrix frauenfeldii) | |||
| [31] | Summer | - Diatoms, Cryptophytes, large Dinoflagellates | ||
| Toulon (France) | [15] | Annual cycle | February/April | - Diatoms (Cyclotella sp., Navicula sp.; Licmophora gracilis, Coscinodiscus sp.); |
| June | - Dinoflagellates (Prorocentrum compressum, Gymnodinium sp.); | |||
| Autumn | - Diatoms (Navicula sp., Coscinodiscus sp., Chaetoceros sp., Cylindrotheca closterium, Cyclotella sp.) | |||
| Villefranche-sur-Mer (France) | [20] | Annual cycle | March/April | - Prymnesiophyceae, Chrysophyceae |
| November | - Diatoms | |||
| [32] | May | - Dinoflagellates (Ceratium furca, Prorocentrum micans, Prorocentrum sp.); | ||
| August/September | - Diatoms (Thalassionema frauenfeldii), Dictyochophyceae (Dictyocha fibula); | |||
| [33] | October/November | - Diatoms (Chaetoceros sp.); | ||
| Summer/Autumn | - Diatoms (Dactyliosolen fragilissimus, Leptocylindrus danicus, L. minimus) | |||
| Naples (Italy) | [34] | Annual cycle | May/June | - Diatoms (Cylindrotheca closterium, Chaetoceros compressus, Nitzschia longissima); |
| October | - Dinoflagellates, Dictyochophyceae (Emiliana huxleyi); | |||
| Winter | - Dictyochophyceae (Emiliana huxleyi) | |||
| [9] | Autumn | November | -Diatoms (Thalassiosira sp., Chaetoceros sp.) | |
| [35] | Annual cycle | Winter/spring | - Diatoms (Chaetoceros compressus, C. didymus, Pseudo-nitzschia delicatissima, Thalassionema bacillaris) | |
| Summer | - Diatoms (Skeletonema pseudocostatum, Chaetoceros tenuissimus, C. socialis) Dinoflagellates (Heterocapsa niei, Prorocentrum triestinum) | |||
| Autumn | - Diatoms (Thalassiosira rotula, Pseudonitzschia delicatissima, Cylindrotheca closterium, Skeletonema menzelii, Dactyliosolen phuketensis, Pseudo-nitzschia multistriata, Leptocylindrus minimus), Dictyochophyceae |

2. Materials and Methods
2.1. Sampling Strategy
2.2. Physico-Chemical Measurements
2.3. Phytoplankton Analysis
2.4. Zooplankton Analysis
2.5. Data Analysis
3. Results
3.1. Winter–Spring Bloom Dynamic of the Phytoplankton Assemblages at Bastia

3.2. Spatio-Temporal Variation of Phytoplankton Assemblages at Bastia and Calvi




| Source of Variation | df | MS | Pseudo-F | p-values |
|---|---|---|---|---|
| Site | 1 | 1130.1 | 6.8807 | 0.0034 |
| Season | 1 | 4768.9 | 29.036 | 0.0001 |
| Site × Season | 1 | 214.81 | 1.3079 | 0.2487 |
| Residuals | 20 | 164.24 | ||
| Total | 23 |
| S1 (Calvi) vs. S2 (Bastia) | ||
|---|---|---|
| Season | t | p-values |
| winter/spring | 2.0266 | 0.0051 |
| summer/autumn | 2.0884 | 0.0033 |

3.3. Abiotic Drivers of Phytoplankton Assemblages
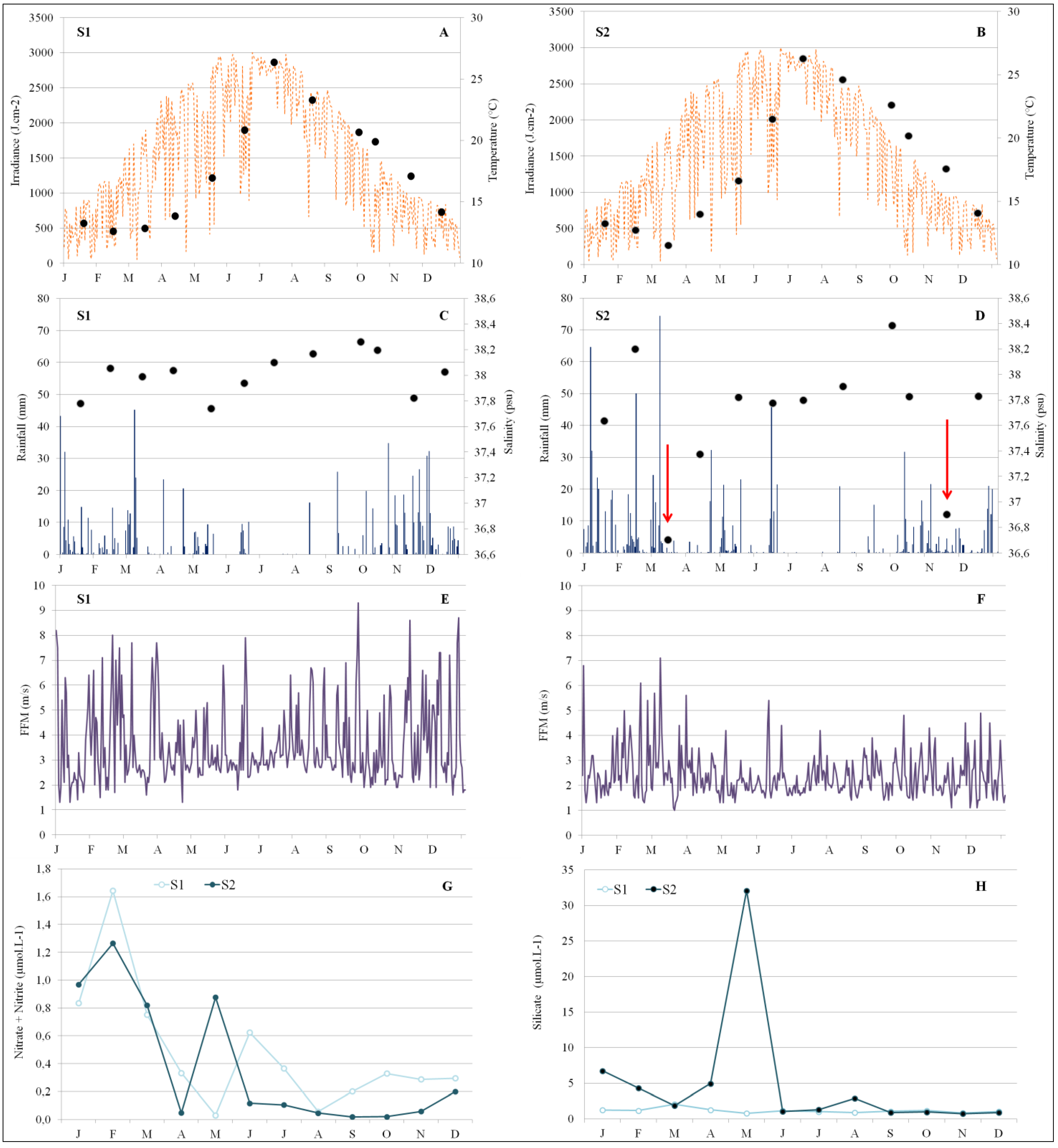

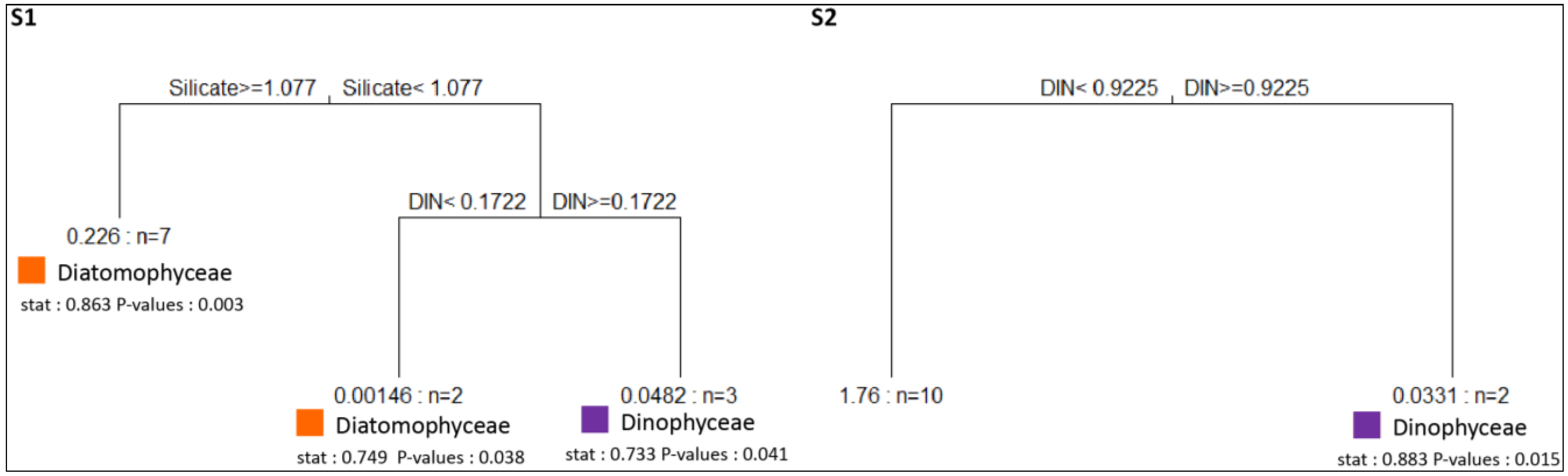
3.4. Zooplankton Variation

4. Discussion-Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Béthoux, J.P.; Gentili, B.; Morin, P.; Nicolas, E.; Pierre, C.; Ruiz-Pino, D. The Mediterranean Sea: A miniature ocean for climatic and environmental studies and a key for the climatic functioning of the North Atlantic. Prog. Oceanogr. 1999, 44, 131–146. [Google Scholar] [CrossRef]
- Krom, M.D.; Kress, N.; Brenner, S.; Gordon, L. Phosphorous limitation of primary productivity in the Eastern Mediterranean Sea. Limnol. Oceanogr. 1991, 36, 424–432. [Google Scholar] [CrossRef]
- Millot, C. Circulation in the Western Mediterranean Sea. J. Mar. Syst. 1999, 20, 423–442. [Google Scholar] [CrossRef]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Anderson, A.; Haecky, P.; Hagstroem, A. Effect of temperature and light on the growth of micro, nano and picoplankton: Impact on algal succession. Mar. Biol. 1994, 120, 511–520. [Google Scholar] [CrossRef]
- Estrada, M. Phytoplankton assemblages across a NW Mediterranean front: Changes from winter mixing to spring stratification. Oecol. Aquat. 1991, 10, 157–185. [Google Scholar]
- Estrada, M.; Varela, R.; Salat, J.; Cruzado, A.; Arias, E. Spatio-temporal variability of the winter phytoplankton distribution across the Catalan and North Balearic fronts (NW Mediterranean). J. Plankton Res. 1999, 21, 1–20. [Google Scholar] [CrossRef]
- Goffart, A.; Hecq, J.H.; Legendre, L. Changes in the development of the winter-spring phytoplankton bloom in the Bay of Calvi (NW Mediterranean) over the last two decades: A response to changing climate? Mar. Ecol. Prog. Ser. 2002, 236, 45–60. [Google Scholar] [CrossRef]
- Zingone, A.; Casotti, R.; Rivera d’Alcala, M.; Scardi, M.; Marino, D. St Martin’s summer: The case of an autumn phytoplankton bloom in the Gulf of Naples (Mediterranean Sea). J. Plankton Res. 1995, 17, 575–593. [Google Scholar] [CrossRef]
- Estrada, M. Primary production in the northwestern Mediterranean. Sci. Mar. 1996, 60, 55–64. [Google Scholar]
- Andersen, V.; Prieur, L. One-month study in the open NW Mediterranean Sea (DYNAPROC experiment, May 1995): Overview of the hydrobiogeochemical structures and effects of wind events. Deep-Sea Res. Part I 2000, 47, 397–422. [Google Scholar] [CrossRef]
- Vilicic, D.; Bosak, S.; Buric, Z.; Caput-Mihalic, K. Phytoplankton seasonality and composition along the coastal NE Adriatic Sea during the extremely low Po River discharge in 2006. Acta Bot. Croat. 2007, 66, 101–115. [Google Scholar]
- Travers, M. Diversité du microplancton du Golfe de Marseille en 1964. Mar. Biol. 1971, 8, 308–343. (In French) [Google Scholar] [CrossRef]
- Charles, F.; Lantoine, F.; Brugel, S.; Chrétiennot-Dinet, M.J.; Quiroga, I.; Rivière, B. Seasonal survey of the phytoplankton biomass, composition and production in a littoral NW Mediterranean site, with special emphasis on the picoplanktonic contribution. Est. Coast. Shelf Sci. 2005, 65, 199–212. [Google Scholar] [CrossRef]
- Jamet, J.L.; Jean, N.; Bogé, G.; Richard, S.; Jamet, D. Plankton succession and assemblage structure in two neighbouring littoral ecosystems in the north-west Mediterranean Sea. Mar. Freshw. Res. 2005, 56, 69–83. [Google Scholar] [CrossRef]
- Polat, S.; Akiz, A.; Olgunoğlu, M.P. Daily variations of coastal phytoplankton assemblages in summer conditions of the northeastern Mediterranean (Bay of İskenderun). Pak. J. Bot. 2005, 37, 715–724. [Google Scholar]
- D’Ortenzio, F.; Ribera d’Alcalà, M. On the trophic regimes of the Mediterranean Sea: A satellite analysis. Biogeosciences 2009, 6, 139–148. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera d’Alcala, M.; Vaqué, D.; Zingone, A. Plankton in the open Mediterranean Sea: A review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef] [Green Version]
- Gohin, F. Annual cycles of Chlorophyll-a, non-algal suspended particulate matter and turbidity observed from space and in situ coastal waters. Ocean Sci. 2011, 7, 705–732. [Google Scholar] [CrossRef]
- Smayda, T.J.; Reynolds, C.S. Community assembly in marine phytoplankton: Application of recent models to harmful dinoflagellates blooms. J. Plankton Res. 2001, 23, 447–461. [Google Scholar] [CrossRef]
- Goffart, A.; Hecq, J.H.; Prieur, L. Contrôle du phytoplancton du bassin Ligure par le front Liguro-Provençal (secteur Corse). Oceanol. Acta 1995, 18, 329–342. (In French) [Google Scholar]
- Fouilland, E.; Courties, C.; Descolas-Gros, C. Size-fractionated carboxylase activities during a 32h cycle at 30 m depth in the north-western Mediterranean Sea after an episodic wind event. J. Plankton Res. 2001, 23, 623–632. [Google Scholar] [CrossRef]
- Pinazo, C.; Marsaleix, P.; Millet, B.; Estournel, C.; Kondrachoff, V.; Vehil, R. Phytoplankton variability in summer in the Northwestern Mediterranean: Modelling of the wind and freshwater impacts. J. Coast. Res. 2001, 17, 146–161. [Google Scholar]
- Spatharis, S.; Tsirtsis, G.; Danielidis, D.B.; Do Chi, T.; Mouillot, D. Effects of pulsed nutrient inputs on phytoplankton assemblage structure and blooms in an enclosed coastal area. Est. Coast. Shelf Sci. 2007, 73, 807–815. [Google Scholar] [CrossRef]
- Bustillos-Guzman, J.; Claustre, H.; Marty, J.C. Specific phytoplankton signatures and their relationship to hydrographic conditions in the coastal northwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 1995, 124, 247–258. [Google Scholar]
- Andersen, V.; Nival, P.; Caparroy, P.; Gubanova, A. Zooplankton community during the transition from spring bloom to oligotrophy in the open NW Mediterranean and effects of wind events. 1. Abundance and specific composition. J. Plankton Res. 2001, 23, 227–242. [Google Scholar] [CrossRef]
- Vidal, M.; Duarte, C.M. Nutrient accumulation at different supply rates in experimental Mediterranean planktonic communities. Mar. Ecol. Prog. Ser. 2000, 207, 1–11. [Google Scholar]
- Béthoux, J.P.; Morin, P.; Ruiz-Pino, D.P. Temporal trends in nutrient ratios: Chemical evidence of Mediterranean ecosystem changes driven by human activity. Deep-Sea Res. Part II 2002, 49, 2007–2016. [Google Scholar] [CrossRef]
- Spatharis, S.; Tsirtsis, G. Ecological quality scales based on phytoplankton for the implementation of Water Framework Directive in the Eastern Mediterranean. Ecol. Indic. 2010, 10, 840–847. [Google Scholar] [CrossRef]
- Jacques, G. Aspects quantitatifs du phytoplancton de Banyuls sur Mer (Golfe du Lion). III Diatomées et Dinoflagellés de juin 1965 à juin 1968. Vie et Milieu 1968, 20, 91–126. (In French) [Google Scholar]
- Thyssen, M.; Beker, B.; Ediger, D.; Yilmaz, D.; Garcia, N.; Denis, M. Phytoplankton distribution during two contrasted summers in a Mediterranean harbour: Combining automated submersible flow cytometry with conventional techniques. Environ. Monit. Assess. 2011, 173, 1–16. [Google Scholar]
- Gomez, F.; Gorsky, G. Annual microplankton cycles in Villefranche Bay, Ligurian Sea, NW Mediterranean. J. Plankton Res. 2003, 25, 323–339. [Google Scholar] [CrossRef]
- Marty, J.C.; Garcia, N.; Raimbault, P. Phytoplankton dynamics and primary production under late summer conditions in the NW Mediterranean Sea. Deep-Sea Res. Part. I 2008, 55, 1131–1149. [Google Scholar] [CrossRef]
- Carrada, G.C.; Hopkins, T.S.; Bonaduce, G.; Ianora, A.; Marino, D.; Modigh, M.; Ribera d’Alcalà, M.; Scotto di Carlo, B. Variability in the hydrographic and biological features of the Gulf of Naples. Mar. Ecol. 1980, 1, 105–120. [Google Scholar] [CrossRef]
- Ribera d’Alcalà, M.; Conversano, F.; Corato, F.; Licandro, P.; Mangoni, O.; Marina, D.; Mazzocchi, M.G.; Modigh, M.; Montresor, M.; Nardella, M.; et al. Seasonal patterns in plankton communities in a pluriannual time series at a coastal Mediterranean site (Gulf of Naples): An attempt to discern recurrences and trends. Sci. Mar. 2004, 68, 65–83. [Google Scholar]
- Pasqualini, V.; Pergent-Martini, C.; Clabaut, P.; Pergent, G. Mapping of Posidonia oceanica using Aerial photographs and Side Scan Sonar: Application off the Island of Corsica (France). Est. Coast. Shelf Sci. 1998, 47, 359–367. [Google Scholar] [CrossRef]
- Mouillot, D.; Titeux, A.; Migon, C.; Sandroni, V.; Frodello, J.P.; Viale, D. Anthropogenic influences on a mediterranean Nature Reserve: Modelling and forecasting. Environ. Model. Assess. 2000, 5, 185–192. [Google Scholar] [CrossRef]
- Garrido, M. Structure et fonction des communautés phytoplanctoniques en milieux côtiers marin et lagunaire (Méditerranée–Corse) dans une optique de gestion. Ph.D. Thesis, University of Corsica, Corte, France, University of Liège, Liège, Belgium, 2012. [Google Scholar]
- Lafabrie, C.; Garrido, M.; Cecchi, P.; Leboulanger, C.; Gregori, G.; Pasqualini, V.; Pringault, O. Impact of contaminated sediment resuspension on phytoplankton in a Mediterranean lagoon: Functional and structural responses. Est. Coast. Shelf Sci. 2013, 130, 70–80. [Google Scholar] [CrossRef]
- Goffart, A. Influence des contraintes hydrodynamiques sur la structure des communautés phytoplanctoniques du bassin Liguro-Provençal (secteur Corse). Ph.D. Thesis, University of Liège, Liège, Belgium, 1992; p. 163. [Google Scholar]
- Beker, B.; Romano, J.C.; Arlhac, D. Le suivi de la variabilité des peuplements phytoplanctoniques en milieu marin littoral: Le golf de Marseille en 1996–1997. Océanis 2001, 25, 395–415. (In French) [Google Scholar]
- Tréguer, P.; LeCorre, P. Manuel d’analyse des sels nutritifs dans l’eau de mer. Utilisation de l’AutoAnalyser II Technicon, 2nd ed.; University Bretagne Occidentale: Laboratoire de Chimie Marine, Brest, France, 1975; pp. 1–110. (In French) [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplanktonmethodik, Mitteilungen der internationalen Vereinigung fur Theoretische and Angewandte. Limnologie 1958, 9, 1–38. (In French) [Google Scholar]
- Association Française de Normalisation (AFNOR). Qualité de l'eau—Norme Guide Pour Le Dénombrement Du Phytoplancton Par Microscopie Inversée (Méthode Utermöhl); AFNOR: Saint-Denis, France, 2006; p. 39. (In French) [Google Scholar]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Uehlinger, V. Etude statistique des méthodes de dénombrement planctonique. Arch. Des Sci. 1964, 17, 11–223. [Google Scholar]
- Jeffrey, S.W.; Mantoura, R.F.C.; Bjørnland, T. Data for the identification of 47 key phytoplankton pigments. In Phytoplankton Pigments in Oceanography; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO (United Nations Educational, Scientific and Cultural Organization): Paris, France, 1997; pp. 447–559. [Google Scholar]
- Zapata, M.; Rodriguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Sieburth, J.M.; Smetacek, V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Claustre, H. The trophic status of various oceanic provinces as revealed by phytoplankton pigment signatures. Limnol. Oceanogr. 1994, 39, 1206–1210. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.C. Phytoplankton pigment distribution in relation to upper thermocline circulation in the eastern Mediterranean Sea during winter. J. Geophys. Res. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Ter Braak, C.J.F. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory, PRIMER-E Limited.: Plymouth, UK, 2001; p. 177. [Google Scholar]
- De’ath, G. Multivariate Regression Trees: A New Technique for Modeling Species-Environment Relationships. Ecology 2002, 83, 1105–1117. [Google Scholar]
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, Z. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- De’ath, G. Mvpart: Multivariate Partitioning, R package version 1.6-1; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. Perspect. Mar. Biol. 1958, 323–349. [Google Scholar]
- Wyatt, T. Margalef’s mandala and phytoplankton bloom strategies. Deep-Sea Res. Part. II 2014, 101, 32–49. [Google Scholar] [CrossRef]
- Smayda, T.J. What is a bloom? A commentary. Limnol. Oceanogr. 1997, 42, 1132–1136. [Google Scholar] [CrossRef]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. J. Cons. Perm. Int. Explor. Mer. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Lévy, M.; Mémery, L.; André, J.M. Simulation of primary production and export fluxes in the Northwestern Mediterranean Sea. J. Mar. Res. 1998, 56, 197–238. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agusti, S.; Kennedy, H.; Vaqué, D. The Mediterranean climate as a template for Mediterranean marine ecosystems: The example of the northeast Spanish littoral. Progr. Oceanogr. 1999, 44, 245–270. [Google Scholar] [CrossRef]
- Delgado, M.; Latasa, M.; Estrada, M. Variability in the size-fractionated distribution of the phytoplankton across the Catalan front of the north-west Mediterranean. J. Plankton Res. 1992, 14, 753–771. [Google Scholar] [CrossRef]
- Mercado, J.M.; Ramirez, T.; Cortés, T.; Sebastian, T.; Vargas-Yaez, M. Seasonal and inter-annual variability of the phytoplankton communities in an upwelling area of the Alboran Sea (SW Mediterranean Sea). Sci. Mar. 2005, 69, 451–465. [Google Scholar]
- Brzezinski, M.A. The Si-C-N ratio of marine diatoms-interspecific variability and the effect of some environmental variables. J. Phycol. 1985, 21, 347–357. [Google Scholar] [CrossRef]
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Agawin, N.S.R.; Duarte, C.M.; Agusti, S. Growth and abundance of Synechococcus. sp. in a Mediterranean Bay: Seasonality and relationship with temperature. Mar. Ecol. Prog. Ser. 1998, 170, 45–53. [Google Scholar] [CrossRef]
- Agusti, S.; Duarte, C.M. Experimental induction of a large phytoplankton bloom in Antarctic coastal waters. Mar. Ecol. Prog. Ser. 2000, 206, 73–85. [Google Scholar] [CrossRef]
- Savenkoff, C.; Prieur, L.; Reys, J.P.; Lefevre, D.; Dallot, S.; Denis, M. Deep microbial communities evidenced in the Liguro-Provençal front by their ETS activity. Deep-Sea Res. Part I 1993, 40, 709–725. [Google Scholar] [CrossRef]
- Gorsky, G.; Lins da Silva, N.; Dallot, S.; Laval, P.; Braconnot, J.C.; Prieur, L. Midwater tunicates: Are they related to the permanent front of the Ligurian Sea (N.W. Mediterranean Sea)? Mar. Ecol. Prog. Ser. 1991, 74, 195–204. [Google Scholar] [CrossRef]
- Brohée, M.; Goffart, A.; Frankignoulle, M.; Henri, V.; Mouchet, A.; Hecq, J.H. Variations printanières des communautés planctoniques en Baie de Calvi (Corse) en relation avec les contraintes physiques locales. Cah. Biol. Mar. 1989; 30, 321–328. (In French) [Google Scholar]
- Quinlan, E.L.; Phlips, E.J. Phytoplankton assemblages across the marine to low-salinity transition zone in a blackwater dominated estuary. J. Plankton Res. 2006, 29, 401–416. [Google Scholar] [CrossRef]
- Brayner, R.; Couté, A.; Livage, J.; Perrette, C.; Sicard, C. Micro-algal biosensors. Anal. Bioanal. Chem. 2011, 401, 581–597. [Google Scholar] [CrossRef]
- Gailhard, I.; Durbec, J.P.; Beliaeff, B.; Sabatier, R. Ecologie du phytoplancton sur les côtes françaises: Comparaison inter-sites. C. R. Biol. 2003, 326, 853–863. [Google Scholar] [CrossRef]
- Li, W. Macroecological patterns of phytoplankton in the northwestern North Atlantic Ocean. Nature 2002, 419, 154–157. [Google Scholar] [CrossRef]
- Bel-Hassen, M.; Hamza, A.; Drira, Z.; Zouari, A.; Akrout, F.; Messaoudi, S.; Aleya, L.; Ayadi, H. Phytoplankton-pigment signatures and their relationship to spring–summer stratification in the Gulf of Gabes. Est. Coast. Shelf Sci. 2009, 83, 296–306. [Google Scholar] [CrossRef]
- Margalef, R. Environmental control of the mesoscale distribution of primary producers and its bearing to primary production in the western Mediterranean. In Mediterraneum. Marine Ecosystems; Moraitou-Apostolopoulou, M., Kiortsis, V., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 213–229. [Google Scholar]
- Lasternas, S.; Tunin-Ley, A.; Ibanez, F.; Andersen, V.; Pizay, M.D.; Lemée, R. Short-term dynamics of microplankton abundance and diversity in NW Mediterranean Sea during late summer conditions (DYNAPROC 2 cruise, 2004). Biogeosciences 2011, 8, 743–761. [Google Scholar] [CrossRef] [Green Version]
- Cloern, J.E.; Dufford, R. Phytoplankton community ecology: Principles applied in San Francisco Bay. Mar. Ecol. Prog. Ser. 2005, 285, 11–28. [Google Scholar] [CrossRef]
- D’Ortenzio, F. Space and Time Occurrence of Algal Blooms in the Mediterranean: Their Significance for the Trophic Regime of the Basin. Ph.D. Thesis, University of London, London, UK, 2003; p. 302. [Google Scholar]
- Puigserve, M.; Monerris, N.; Pablo, J.; Alos, J.; Moya, G. Abundance patterns of the toxic phytoplankton in coastal waters of the Balearic Archipelago (NW Mediterranean Sea): A multivariate approach. Hydrobiologia 2010, 644, 145–157. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Patterns and scales of phytoplankton variability in estuarine coastal ecosystems. Est. Coast. 2009, 33, 230–241. [Google Scholar]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Crane, K.W.; Grover, J.P. Coexistence of mixotrophs, autotrophs, and heterotrophs in planktonic microbial communities. J. Theor. Biol. 2010, 262, 517–527. [Google Scholar] [CrossRef]
- Henriksen, P.; Revilla, M.; Lehtinen, S.; Kauppila, P.; Kaitala, S.; Agusti, S.; Icely, J.; Basset, A.; Moncheva, S.; Sørensen, K. Deliverable D4.1-2: Assessment of pigment data potential for multi-species and assemblage indices. Available online: http://www.wiser.eu/download/D4.1-2.pdf (accessed on 27 March 2014).
- Domingues, R.B.; Barbosa, A.; Galvão, H. Constraints on the use of phytoplankton as a biological quality element within the Water Framework Directive in Portuguese waters. Mar. Pollut. Bull. 2008, 56, 1389–1395. [Google Scholar] [CrossRef]
- Devlin, M.; Barry, J.; Painting, S.; Best, M. Extending the phytoplankton tool kit for the UK Water Framework Directive: Indicators of phytoplankton community structure. Hydrobiologia 2009, 633, 151–168. [Google Scholar] [CrossRef]
- Carletti, A.; Heiskanen, A.S. Water Framework Directive Intercalibration Technical Report. Part 3: Coastal and Transitional Waters. In JRC Scientific and Technical Reports; European Communities: Luxembourg, 2009; p. 244. [Google Scholar]
- Sherrard, N.J.; Nimmo, M.; Llewellyn, C.A. Combining HPLC pigment markers and ecological similarity indices to assess phytoplankton community structure: An environmental tool for eutrophication? Sci. Total Environ. 2006, 361, 97–110. [Google Scholar] [CrossRef]
- Sarmento, H.; Descy, J. Use of marker pigments and functional groups for assessing the status of phytoplankton assemblages in lakes. J. Appl. Phycol. 2008, 20, 1001–1011. [Google Scholar] [CrossRef]
- Van Lenning, K.; Latasa, M.; Estrada, M.; Saéz, A.G.; Medlin, L.; Probert, I.; Véron, B.; Young, J. Pigments signatures phylogenetic relationships of the Pavlophyceae (Haptophyta). J. Phycol. 2003, 39, 379–389. [Google Scholar] [CrossRef]
- Zapata, M.; Edvardsen, B.; Rodríguez, F.; Maestro, M.A.; Garrido, J.L. Chlorophyll c2 monogalactosyldiacylglyceride ester (chl c2-MGDG). A novel marker pigment for Chrysochromulina species (Haptophyta). Mar. Ecol. Prog. Ser. 2001, 219, 85–98. [Google Scholar] [CrossRef]
- Zapata, M.; Garrido, J.L.; Jeffrey, S.W. Chlorophyll c pigments: Current status. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Porra, R.J., Rüdiger, W., Scheer, U., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 39–53. [Google Scholar]
- Latasa, M.; Scharek, R.; Le Gall, F.; Guillou, L. Pigment suites and taxonomic groups in Prasinophyceae. J. Phycol. 2004, 40, 1149–1155. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garrido, M.; Koeck, B.; Goffart, A.; Collignon, A.; Hecq, J.-H.; Agostini, S.; Marchand, B.; Lejeune, P.; Pasqualini, V. Contrasting Patterns of Phytoplankton Assemblages in Two Coastal Ecosystems in Relation to Environmental Factors (Corsica, NW Mediterranean Sea). Diversity 2014, 6, 296-322. https://doi.org/10.3390/d6020296
Garrido M, Koeck B, Goffart A, Collignon A, Hecq J-H, Agostini S, Marchand B, Lejeune P, Pasqualini V. Contrasting Patterns of Phytoplankton Assemblages in Two Coastal Ecosystems in Relation to Environmental Factors (Corsica, NW Mediterranean Sea). Diversity. 2014; 6(2):296-322. https://doi.org/10.3390/d6020296
Chicago/Turabian StyleGarrido, Marie, Barbara Koeck, Anne Goffart, Amandine Collignon, Jean-Henri Hecq, Sylvia Agostini, Bernard Marchand, Pierre Lejeune, and Vanina Pasqualini. 2014. "Contrasting Patterns of Phytoplankton Assemblages in Two Coastal Ecosystems in Relation to Environmental Factors (Corsica, NW Mediterranean Sea)" Diversity 6, no. 2: 296-322. https://doi.org/10.3390/d6020296




