Citric Acid Injections: An Accessible and Efficient Method for Controlling Outbreaks of the Crown-of-Thorns Starfish Acanthaster cf. solaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection Site and Maintenance Conditions
2.2. Experiment 1: Concentration and Number of Injection Sites
2.3. Experiment 2: Volume
2.4. Experiment 3: Seawater vs Distilled Water
2.5. Statistical Analysis
3. Results
3.1. Experiment 1: Concentration and Number of Injection Sites
Behaviour and Macroscopic Progression
3.2. Experiment 2: Volume
3.3. Experiment 3: Water Type
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birkeland, C.; Lucas, J. Acanthaster planci: Major Management Problem of Coral Reefs; CRC press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Ann. Rev. 2014, 52, 133–200. [Google Scholar]
- Chesher, R.H. Destruction of Pacific corals by the sea star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Johns, K.A. Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef] [PubMed]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Nat. Acad. Sci. 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Owens, L.; Caballes, C.F.; Pratchett, M.S. The role of protein extracts in the induction of disease in Acanthaster planci. J. Exp. Mar. Biol. Ecol. 2012, 429, 1–6. [Google Scholar] [CrossRef]
- Hughes, T.P.; Graham, N.A.; Jackson, J.B.; Mumby, P.J.; Steneck, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Pratchett, M.S. A review of existing control efforts for Acanthaster planci; limitations to successes. Report to the Department of Sustainability, Environment, Water, Population & Communities, NERP, Tropical Environmental Hub: Townsville, Australia, 2012. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.721.3890&rep=rep1&type=pdf (accessed on 20 April 2015).
- Rivera-Posada, J.; Caballes, C.F.; Pratchett, M.S. Lethal doses of oxbile, peptones and thiosulfate-citrate-bile-sucrose agar (TCBS) for Acanthaster planci; exploring alternative population control options. Mar. Poll. Bull. 2013, 75, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Pratchett, M.S.; Aguilar, C.; Grand, A.; Caballes, C.F. Bile salts and the single-shot lethal injection method for killing crown-of-thorns sea stars (Acanthaster planci). Ocean Coast. Manag. 2014, 102, 383–390. [Google Scholar] [CrossRef]
- Crown-of-thorns starfish control guidelines. Great Barrier Reef Marine Park Authority: Townsville, Australia, 2014. Available online: http://hdl.handle.net/11017/2874 (accessed on 20 April 2015).
- Boström-Einarsson, L.; Rivera-Posada, J. Controlling outbreaks of the coral-eating crown-of-thorns starfish using a single injection of common household vinegar. Coral Reefs 2016, 35, 223–228. [Google Scholar] [CrossRef]
- De Dios, H.Y.; Sotto, B.F.; Dy, D.T.; Ilano, A.S. Response of Acanthaster planci (Echinodermata: Asteroidea) to hypersaline solution: Its potential application to population control. Galaxea 2015, 17, 23–30. [Google Scholar] [CrossRef]
- Introduction to the Control of COTS by Acetic Acid Injection. Kuroshio Biological Research Foundation: Kochi, Japan, 2012. Available online: https://chushikoku.env.go.jp/to_2012/data/0530aa_2.pdf (accessed on 23 June 2015).
- Moutardier, G.; Gereva, S.; Mills, S.C.; Adjeroud, M.; Beldade, R.; Ham, J.; Kaku, R.; Dumas, P. Lime Juice and Vinegar Injections as a Cheap and Natural Alternative to Control COTS Outbreaks. PLoS ONE 2015, 10, e0137605. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Posada, J.; Owens, L. Osmotic shock as alternative method to control Acanthaster planci. J. Coastal Life Med. 2014, 2, 99–106. [Google Scholar] [CrossRef]
- Yamamoto, T.; Otsuka, T. Experimental validation of dilute acetic acid solution injection (Acanthaster planci). Naturalistae 2013, 17, 63–65. [Google Scholar]
- Dumas, P.; Gereva, S.; Moutardier, M.; Ham, J.; Kaku, R. Collective action and lime juice fight crown-of-thorns starfish outbreaks in Vanuatu. SPC Fish. Newsl. 2015, 146, 47–52. Available online: http://www.spc.int/DigitalLibrary/Doc/FAME/InfoBull/FishNews/146/FishNews146_47_Dumas.pdf (accessed on 23 June 2015). [Google Scholar]
- Nichols, D. The water-vascular system in living and fossil echinoderms. Palaeontology 1972, 15, 519–538. [Google Scholar]
- Rivera-Posada, J.; Pratchett, M.S.; Cano-Gómez, A.; Arango-Gómez, J.; Owens, L. Injection of Acanthaster planci with thiosulfate-citrate-bile-sucrose agar (TCBS). I. Disease induction. Dis. Aquat. Organ. 2011, 97, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Rivera-Posada, J.; Aguilar, C.; Caballes, F.C.; Grand, A. Efficacy of Oxbile for Controlling Outbreak Populations of Acanthaster Planci on the Great Barrier Reef; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 2007; pp. 1–8.
- Sweatman, H.; Butler, I. An experimental investigation of the ability of adult crown-of-thorns starfish to survive physical damage. In The Possible Causes and Consequences of Outbreaks of the Crown-of-Thorns Starfish, Proceedings of a Workshop series N° 18, Townsville, QLD, Australia, 10 June 1992; Engelhardt, U., Lassing, B., Eds.; Great Barrier Reef Marine Park Authority: Townsville, Australia, 1993; pp. 71–82. [Google Scholar]
- Messmer, V.; Pratchett, M.S.; Clark, T. Capacity for regeneration in crown of thorns starfish, Acanthaster planci. Coral Reefs 2013, 32, 461. [Google Scholar] [CrossRef]
- Glynn, P.W. Acanthaster population regulation by a shrimp and a worm. In Proceedings of the Fourth International Coral Reef Symposium, Manila, Phillippines, 1981; Gomez, E.D., Birkeland, C.E., Buddemeier, R.W., Johannes, R.E., Marsh, J.A., Tsuda, R.T., Eds.; Marine Sciences Center: Quezon City, Phillippines, 1982; Volume 2, pp. 607–612. [Google Scholar]
- Glynn, P.W. An amphinomid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western Pacific coral reefs. Bull. Mar. Sci 1984, 35, 54–71. [Google Scholar]
- Santos-Gouvea, I.A.; Freire, C.A. Effects of hypo-and hypersaline seawater on the microanatomy and ultrastructure of epithelial tissues of Echinometra lucunter (Echinodermata: Echinoidea) of intertidal and subtidal populations. Zool. Stud. 2007, 46, 203–215. [Google Scholar]
- Wittmann, A.C.; Pörtner, H.O. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Chang. 2013, 3, 995–1001. [Google Scholar] [CrossRef]
- Yamaguchi, M. Acanthaster planci infestations of reefs and coral assemblages in Japan: A retrospective analysis of control efforts. Coral Reefs 1986, 5, 23–30. [Google Scholar] [CrossRef]
- Bos, A.R.; Gumanao, G.S.; Mueller, B.; Saceda-Cardoza, M.M. Management of crown-of-thorns sea star (Acanthaster planci L.) outbreaks: Removal success depends on reef topography and timing within the reproduction cycle. Ocean Coast. Manage. 2013, 71, 116–122. [Google Scholar] [CrossRef]
- Human and Environmental Risk Assessment on ingredients of Household Cleaning Products. Substance: Citric Acid and Salts (CAS# 77–92–9; 5949–29–1; 6132–04–3). 2005. Available online: http://www.heraproject.com/files/37-f-05-hera_citricacid_version1_april05.pdf (accessed on 23 June 2015).
- Hoyt, H.L.; Gewanter, H.L. Citrate. In Detergent; De Oude, N.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 3, pp. 229–242. [Google Scholar] [CrossRef]
- OECD SIDS. Citric Acid CAS N˚77–92–9. 2001. Available online: http://www.inchem.org/documents/sids/sids/77929.pdf (accessed on 23 June 2015).
- Great Barrier Reef Marine Park Authority. Crown-of-thorns starfish control guidelines. GBRMPA: Townsville, Australia, 2014. Available online: http://www.gbrmpa.gov.au/__data/assets/pdf_file/0006/185298/COTS-control-guidelines.pdf (accessed on 23 June 2015). [Google Scholar]
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected with 20 mL of citric acid and seawater solution at concentrations of 90, 120, and 150 g·L−1 in one (i), two (ii) and four (iii) injection sites (I.S.). Numbers above bars represent total percent mortality; where no numbers are shown, mortality was equal to 100%. For the 150 g·L−1, 4 I.S. treatment, time to immobility was assumed to equal time to death (
) ± standard error for Acanthaster cf. solaris injected with 20 mL of citric acid and seawater solution at concentrations of 90, 120, and 150 g·L−1 in one (i), two (ii) and four (iii) injection sites (I.S.). Numbers above bars represent total percent mortality; where no numbers are shown, mortality was equal to 100%. For the 150 g·L−1, 4 I.S. treatment, time to immobility was assumed to equal time to death (  ). Six replicates per treatment combination were used. Letter notations above bars (a, b, c) indicate Tukey’s post-hoc groupings between injection site treatments and concentrations for time to death. Different letters indicate significant differences among treatments.
). Six replicates per treatment combination were used. Letter notations above bars (a, b, c) indicate Tukey’s post-hoc groupings between injection site treatments and concentrations for time to death. Different letters indicate significant differences among treatments.
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected with 20 mL of citric acid and seawater solution at concentrations of 90, 120, and 150 g·L−1 in one (i), two (ii) and four (iii) injection sites (I.S.). Numbers above bars represent total percent mortality; where no numbers are shown, mortality was equal to 100%. For the 150 g·L−1, 4 I.S. treatment, time to immobility was assumed to equal time to death (
) ± standard error for Acanthaster cf. solaris injected with 20 mL of citric acid and seawater solution at concentrations of 90, 120, and 150 g·L−1 in one (i), two (ii) and four (iii) injection sites (I.S.). Numbers above bars represent total percent mortality; where no numbers are shown, mortality was equal to 100%. For the 150 g·L−1, 4 I.S. treatment, time to immobility was assumed to equal time to death (  ). Six replicates per treatment combination were used. Letter notations above bars (a, b, c) indicate Tukey’s post-hoc groupings between injection site treatments and concentrations for time to death. Different letters indicate significant differences among treatments.
). Six replicates per treatment combination were used. Letter notations above bars (a, b, c) indicate Tukey’s post-hoc groupings between injection site treatments and concentrations for time to death. Different letters indicate significant differences among treatments.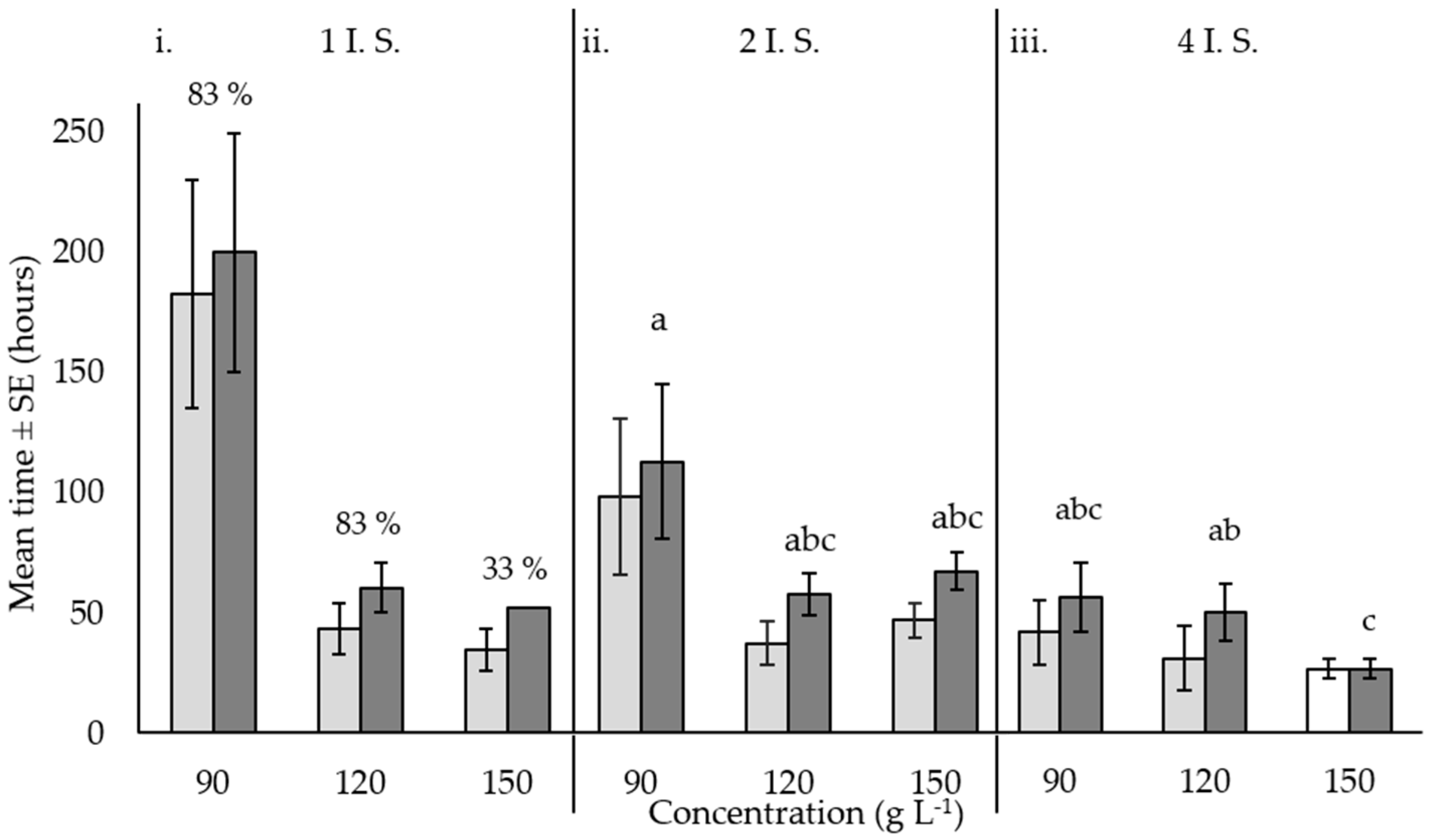
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected in one site with either 20 mL or 30 mL of 120 g·L−1 citric acid and seawater solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
) ± standard error for Acanthaster cf. solaris injected in one site with either 20 mL or 30 mL of 120 g·L−1 citric acid and seawater solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected in one site with either 20 mL or 30 mL of 120 g·L−1 citric acid and seawater solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
) ± standard error for Acanthaster cf. solaris injected in one site with either 20 mL or 30 mL of 120 g·L−1 citric acid and seawater solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.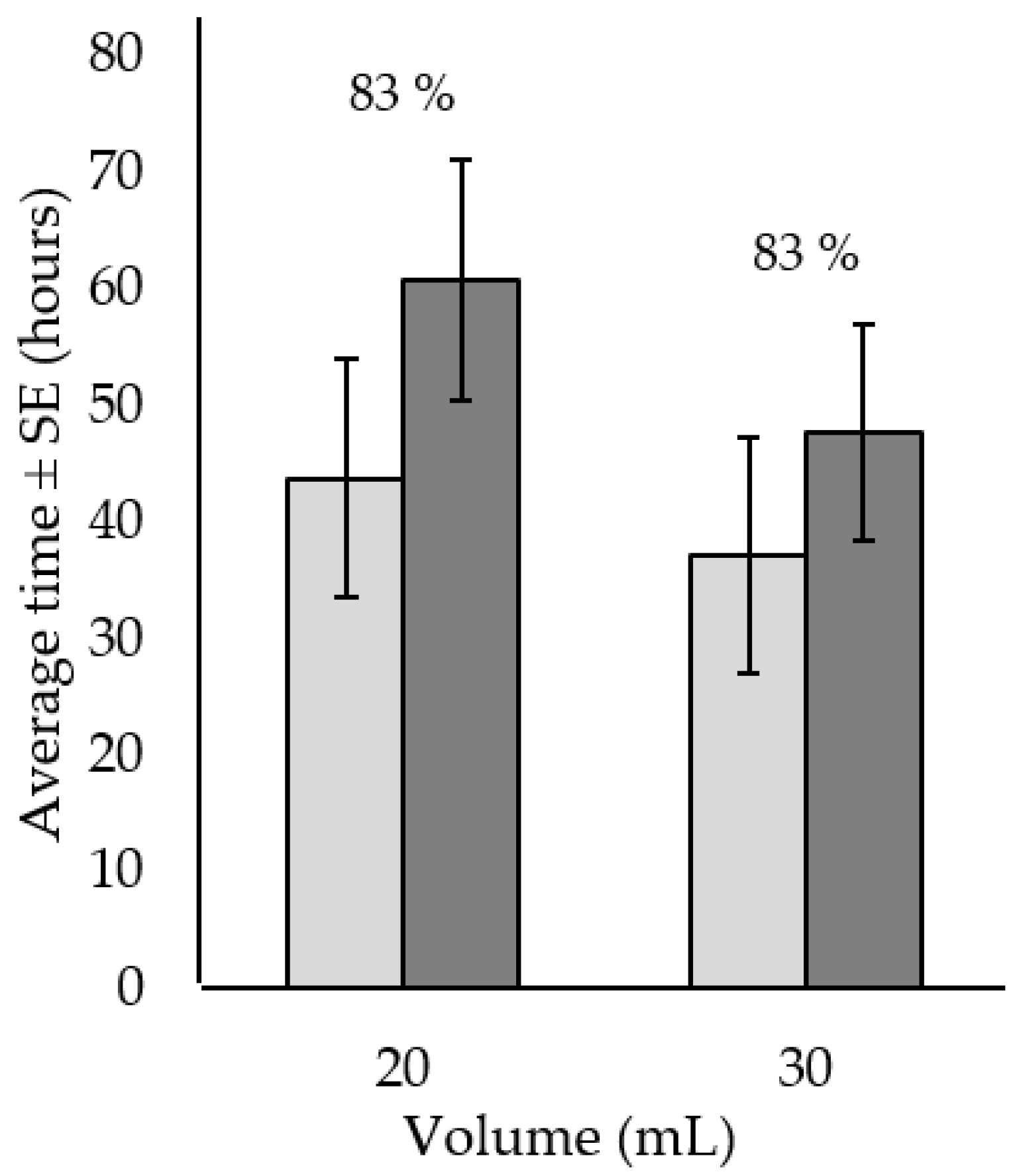
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected in one site with 20 mL of 120 g·L−1 citric acid and either seawater or distilled water solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
) ± standard error for Acanthaster cf. solaris injected in one site with 20 mL of 120 g·L−1 citric acid and either seawater or distilled water solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
 ) and to death (
) and to death (  ) ± standard error for Acanthaster cf. solaris injected in one site with 20 mL of 120 g·L−1 citric acid and either seawater or distilled water solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.
) ± standard error for Acanthaster cf. solaris injected in one site with 20 mL of 120 g·L−1 citric acid and either seawater or distilled water solution. Numbers above bars represent total percent mortality for each treatment. Six replicates per treatment were used.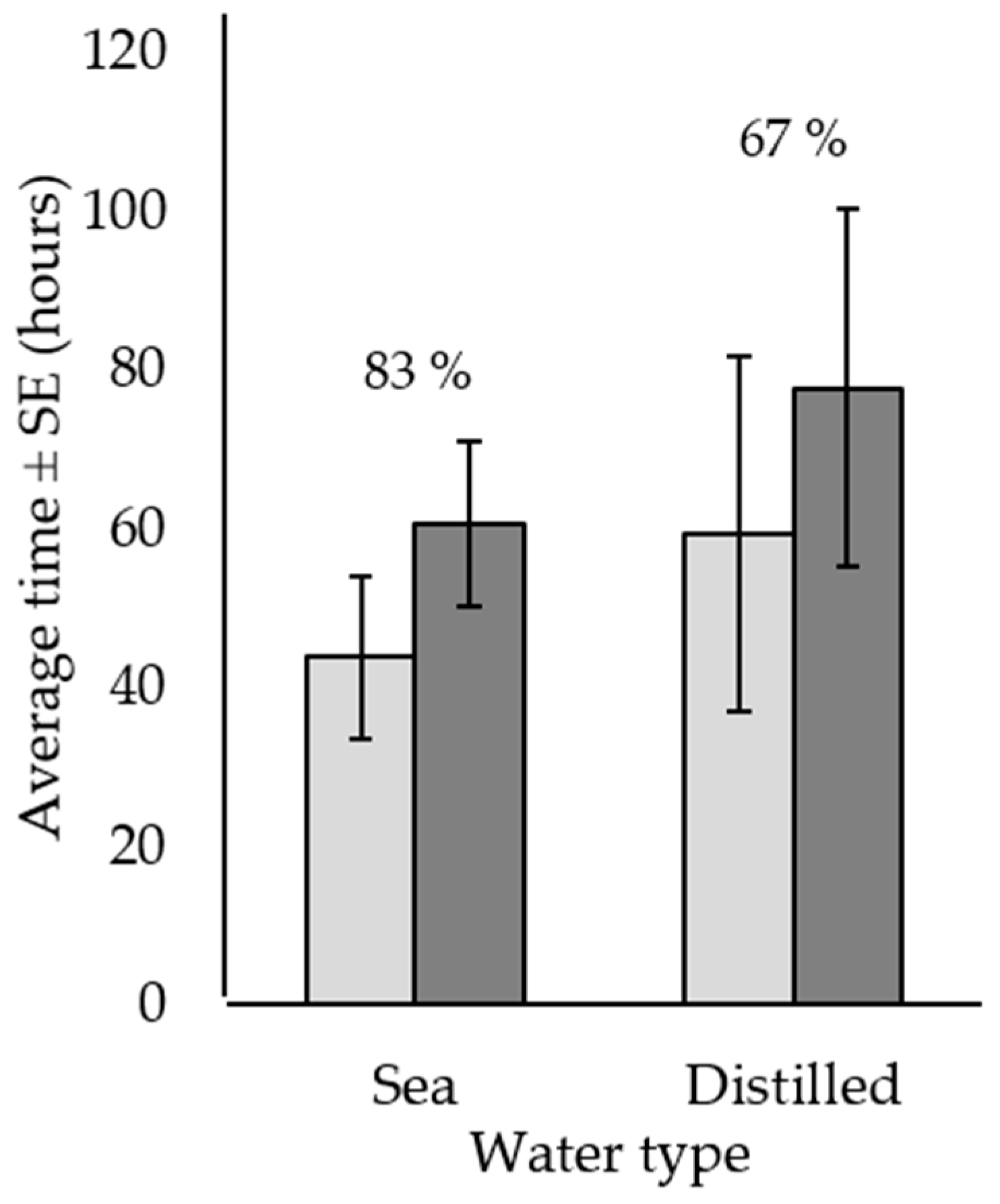
| Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Experiment 1: two-way ANCOVA | |||||
| Log(Time to Immobility) | |||||
| Size | 1 | 0.20 | 0.20 | 0.18 | 0.677 |
| Concentration | 2 | 8.94 | 4.47 | 3.91 | 0.031 * |
| Injection sites | 1 | 1.74 | 1.74 | 1.53 | 0.226 |
| Error | 30 | 34.23 | 1.14 | ||
| Log(Time to Death) | |||||
| Size | 1 | 0.01 | 0.01 | 0.02 | 0.882 |
| Concentration | 2 | 1.35 | 0.68 | 2.49 | 0.100 |
| Injection sites | 1 | 2.82 | 2.82 | 10.37 | 0.003 * |
| Error | 30 | 8.16 | 0.27 | ||
| Experiment 2: one-way ANCOVA | |||||
| Time to Immobility | |||||
| Size | 1 | 196.60 | 196.60 | 0.34 | 0.579 |
| Volume | 1 | 23.44 | 23.44 | 0.04 | 0.847 |
| Error | 7 | 4070.46 | 581.49 | ||
| Time to Death | |||||
| Size | 1 | 709.08 | 709.08 | 1.43 | 0.270 |
| Volume | 1 | 96.74 | 96.74 | 0.20 | 0.671 |
| Error | 7 | 3454.80 | 493.54 | ||
| Experiment 3: one-way ANCOVA | |||||
| Time to Immobility | |||||
| Size | 1 | 61.44 | 61.44 | 0.05 | 0.836 |
| Water type | 1 | 800.55 | 800.55 | 0.61 | 0.465 |
| Error | 6 | 7878.63 | 1313.11 | ||
| Time to Death | |||||
| Size | 1 | 367.11 | 367.11 | 0.30 | 0.603 |
| Water type | 1 | 1241.10 | 1241.10 | 1.01 | 0.352 |
| Error | 6 | 7333.14 | 1222.19 |
| Absolute Lethal Dose (LD100) | Time to Death | Advantages | Disadvantages | |
|---|---|---|---|---|
| Sodium bisulphate | Multiple injections of up to 180 mL [33] of 140 g·L−1 solution [8,9] | Unreported | -Highly effective | -Multiple injections required -Potent oxygen scavenger [8,9] |
| Bile salts | 1 × 10 mL injection of 8 g·L−1 solution [10] | ~ 28 h [10] | -Single injection -No known environmental side effects | -Not readily available in remote areas -Quarantine restrictions on access -<0.05 to 0.29 USD per injection [10,15,18,33] |
| Cooking salt | 2 × 10 mL injections of 400 g·L−1 solution [13,16] | ~ 48 h [16] | -Readily available -No known environmental side effects -<0.05 USD per COTS [13] | -High quantities required (8 kg/1000 COTS) -Solution preparation requires heating -Precipitation and crystallization [16] |
| Vinegar | 2 × 10 mL injections [12,18] or 1 × 25 mL injection [15] | ~30 h [15], ~40 h [12] | -Single injection -Readily available -No known environmental side effects -<0.05 USD per COTS [15] | -High quantities required (20–25 L/1000 COTS) |
| Lime juice | 2 × 10 mL injections [15,18] | ~ 20 h [15] | -No known environmental side effects [15] | -High quantities required (20 L/1000 COTS) -Laborious process for juice extraction -Seasonal and not ubiquitously cheap -Perishable |
| Powdered Citric acid a | 2 × 10 mL or 4 × 5 mL injections of 90–150 g·L−1 solution | ~ 26 h b | -Readily available, long shelf life -No known environmental side effects -<0.05 USD per COTS a -Easily transportable (180–300 g/1000 COTS) | Multiple injections required |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buck, A.C.E.; Gardiner, N.M.; Boström-Einarsson, L. Citric Acid Injections: An Accessible and Efficient Method for Controlling Outbreaks of the Crown-of-Thorns Starfish Acanthaster cf. solaris. Diversity 2016, 8, 28. https://doi.org/10.3390/d8040028
Buck ACE, Gardiner NM, Boström-Einarsson L. Citric Acid Injections: An Accessible and Efficient Method for Controlling Outbreaks of the Crown-of-Thorns Starfish Acanthaster cf. solaris. Diversity. 2016; 8(4):28. https://doi.org/10.3390/d8040028
Chicago/Turabian StyleBuck, Alexander C. E., Naomi M. Gardiner, and Lisa Boström-Einarsson. 2016. "Citric Acid Injections: An Accessible and Efficient Method for Controlling Outbreaks of the Crown-of-Thorns Starfish Acanthaster cf. solaris" Diversity 8, no. 4: 28. https://doi.org/10.3390/d8040028






