Modelling Growth of Juvenile Crown-of-Thorns Starfish on the Northern Great Barrier Reef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Collection
2.2. Growth Curve Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Chlorophyll a (μg·L−1) | Mean Size (mm) | Standard Error (SE) | 95% Confidence Interval (mm) |
|---|---|---|---|
| 0.28 | 0.44 | 0.07 | 0.30–0.58 |
| 2.90 | 0.66 | 0.05 | 0.56–0.76 |
| 5.20 | 0.64 | 0.09 | 0.46–0.82 |
References
- Moran, P.J. The Acanthaster phenomenon. In Oceanography and Marine Biology: An Annual Review; Aberdeen University Press: Townsville, Australia, 1986. [Google Scholar]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Johns, K.A. Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Pratchett, M.S.; Hoey, A.S.; Herdiana, Y.; Campbell, S.J. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 2013, 32, 803–812. [Google Scholar] [CrossRef]
- Chesher, R.H. Destruction of Pacific Corals by the Sea Star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Caballes, C.F.; Posada, J.A.R.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Vanhatalo, J.; Hosack, G.R.; Sweatman, H. Spatiotemporal modelling of crown-of-thorns starfish outbreaks on the Great Barrier Reef to inform control strategies. J. Appl. Ecol. 2016. [Google Scholar] [CrossRef]
- Moore, R.J. Persistent and transient populations of the crown-of-thorns starfish, Acanthaster planci. In Acanthaster and the Coral Reef: A Theoretical Perspective, Proceedings of a Workshop Held at the Australian Institute of Marine Science, Townsville, Australia, 6–7 August 1988; Bradbury, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 236–277. [Google Scholar]
- Pratchett, M.S. Dynamics of an outbreak population of Acanthaster planci at Lizard Island, northern Great Barrier Reef (1995–1999). Coral Reefs 2005, 24, 453–462. [Google Scholar] [CrossRef]
- Zann, L.; Brodie, J.; Berryman, C.; Naqasima, M. Recruitment, ecology, growth and behavior of juvenile Acanthaster Planci (L.) (Echinodermata: Asteroidea). Bull. Mar. Sci. 1987, 41, 561–575. [Google Scholar]
- Caballes, C.F.; Pratchett, M.S. Reproductive biology and early life history of the crown-of-thorns starfish. In Echinoderms—Ecology, Habitats and Reproductive Biology; Whitmore, E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 101–146. [Google Scholar]
- Morello, E.B.; Plagányi, É.E.; Babcock, R.C.; Sweatman, H.; Hillary, R.; Punt, A.E. Model to manage and reduce crown-of-thorns starfish outbreaks. Mar. Ecol. Prog. Ser. 2014, 512, 167–183. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Mellin, C.; Pratchett, M.S.; Hoey, J.; Anthony, K.R.N.; Cheal, A.J.; Miller, I.; Sweatman, H.; Cowan, Z.L.; Taylor, S.; et al. Joint estimation of crown of thorns (Acanthaster planci) densities on the Great Barrier Reef. PeerJ 2016, 4, e2310. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.J.; Davidson, J. Monitoring the distribution and abundance of juvenile Acanthaster planci. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Volume 2, pp. 131–136.
- Pearson, R.G.; Endean, R. A Preliminary Study of the Coral Predator Acanthaster planci (L) (Asteroidea) on the Great Barrier Reef; Fisheries Notes, Department of Harbours and Marine Queensland: Brisbane, Australia, 1969; pp. 27–68. [Google Scholar]
- Johnson, D.B.; Moran, P.J.; Baker, V.J.; Christie, C.A.; Miller, I.R.; Miller-Smith, B.A.; Thompson, A.A. Report on Field Surveys to Locate High Density Populations of Juvenile Crown-of-Thorns Starfish (Acanthaster Planci) within the Central Great Barrier Reef; Australian Institute of Marine Science: Townsville, Australia, 1991. [Google Scholar]
- Habe, T.; Yamamoto, G.; Nagai, A.; Kosaka, M.; Ogura, M.; Sawamoto, S.; Ueno, S.; Yokochi, H. Studies on the Conservation and Management of Coral Reefs and the Control of Acanthaster Planci Juveniles; Report of Grant-in-Aid for Scientific Research, Ministry of Education, Science and Culture; Japan, 1989; pp. 158–186. [Google Scholar]
- Yamaguchi, M. Early life histories of coral reef asteroids, with special reference to Acanthaster planci (L.). In Biology and Geology of Coral Reefs; Jones, O.A., Endean, R., Eds.; Academic Press, Inc.: New York, NY, USA, 1973; pp. 369–387. [Google Scholar]
- Lucas, J.S. Environmental influences on the early development of Acanthaster planci (L.). In Proceedings of the Crown-of-Thorns Starfish Seminar Proceedings, Brisbane, Australia, 6 September 1974; Australian Government Publishing Service: Canberra, Australia, 1974; pp. 109–121. [Google Scholar]
- Yamaguchi, M. Growth of juvenile Acanthaster planci (L.) in the laboratory. Pac. Sci 1974, 28, 123–138. [Google Scholar]
- Lucas, J.S. Growth, maturation and effects of diet in Acanthaster planci (L.) (Asteroidea) and hybrids reared in the laboratory. J. Exp. Mar. Biol. Ecol. 1984, 79, 129–147. [Google Scholar] [CrossRef]
- Henderson, J.A.; Lucas, J.S. Larval development and metamorphosis of Acanthaster planci (Asteroidea). Nature 1971, 232, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Zann, L.; Brodie, J.; Vuki, V. History and dynamics of the crown-of-thorns starfish Acanthaster planci (L.) in the Suva area, Fiji. Coral Reefs 1990, 9, 135–144. [Google Scholar] [CrossRef]
- Okaji, K. Feeding Ecology in the Early Life Stages of the Crown-of-Thorns Starfish, Acanthaster planci (L.). Ph.D. Thesis, James Cook University, Townsville, Australia, February 1996; p. 140. [Google Scholar]
- Kenchington, R.A. Growth and recruitement of Acanthaster planci (L.) on the Great Barrier Reef. Biol. Conserv. 1977, 11, 103–118. [Google Scholar] [CrossRef]
- Haszprunar, G.; Spies, M. An integrative approach to the taxonomy of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): A review of names and comparison to recent molecular data. Zootaxa 2014, 3841, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Doyle, J.; Duggan, S.; Yasuda, N.; McKinnon, A.D. Outbreak of coral-eating Crown-of-Thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S. Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J. Exp. Mar. Biol. Ecol. 1982, 65, 173–193. [Google Scholar] [CrossRef]
- Uthicke, S.; Pecorino, D.; Albright, R.; Negri, A.P.; Cantin, N.; Liddy, M.; Dworjanyn, S.; Kamya, P.; Byrne, M.; Lamare, M. Impacts of ocean acidification on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS ONE 2013, 8, e82938. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.; Hardy, N.; Lamare, M. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, e0122010. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Dworjanyn, S.A.; Mos, B.; Caballes, C.F.; Thompson, C.; Blowes, S. Larval survivorship and settlement of crown-of-thorns starfish (Acanthaster cf. solaris) at varying chlorophyll concentrations. Diversity 2016. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 21. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-128; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2009. [Google Scholar]
- Birkeland, C.; Lucas, J.S. Acanthaster planci: Major Management Problem of Coral Reefs; CRC Press, Inc.: Boca Raton, FL, USA, 1990; p. 257. [Google Scholar]
- Yokochi, H.; Ogura, M. Spawning period and discovery of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea) at northwestern Iriomote-Jima, Ryukyu Islands. Bull. Mar. Sci. 1987, 41, 611–616. [Google Scholar]
- Keesing, J.; Halford, A. Field measurement of survival rates of juvenile Acanthaster planci, techniques and preliminary results. Mar. Ecol. Prog. Ser. 1992, 85, 107–114. [Google Scholar] [CrossRef]
- Australian Institute of Marine Science. The Future of the Reef. Data Centre, AIMS. Available online: http://data.aims.gov.au/aimsrtds/yearlytrends.xhtml (accessed on 22 December 2016).
- Wooldridge, S.A.; Brodie, J.E. Environmental triggers for primary outbreaks of crown-of-thorns starfish on the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2015, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Meteorology (BOM). eReefs. Available online: http://www.bom.gov.au/marinewaterquality/ (accessed on 22 December 2016).

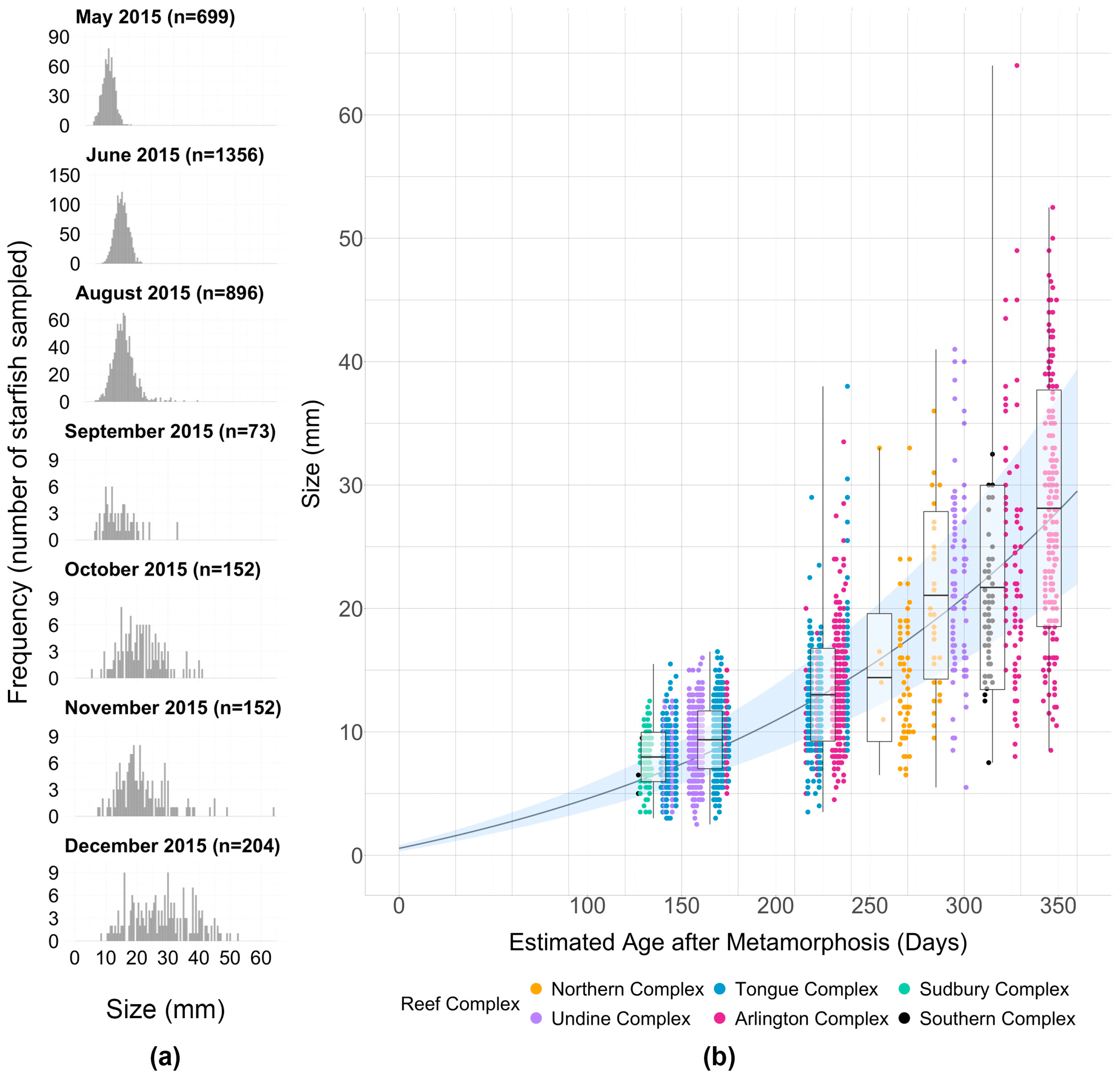
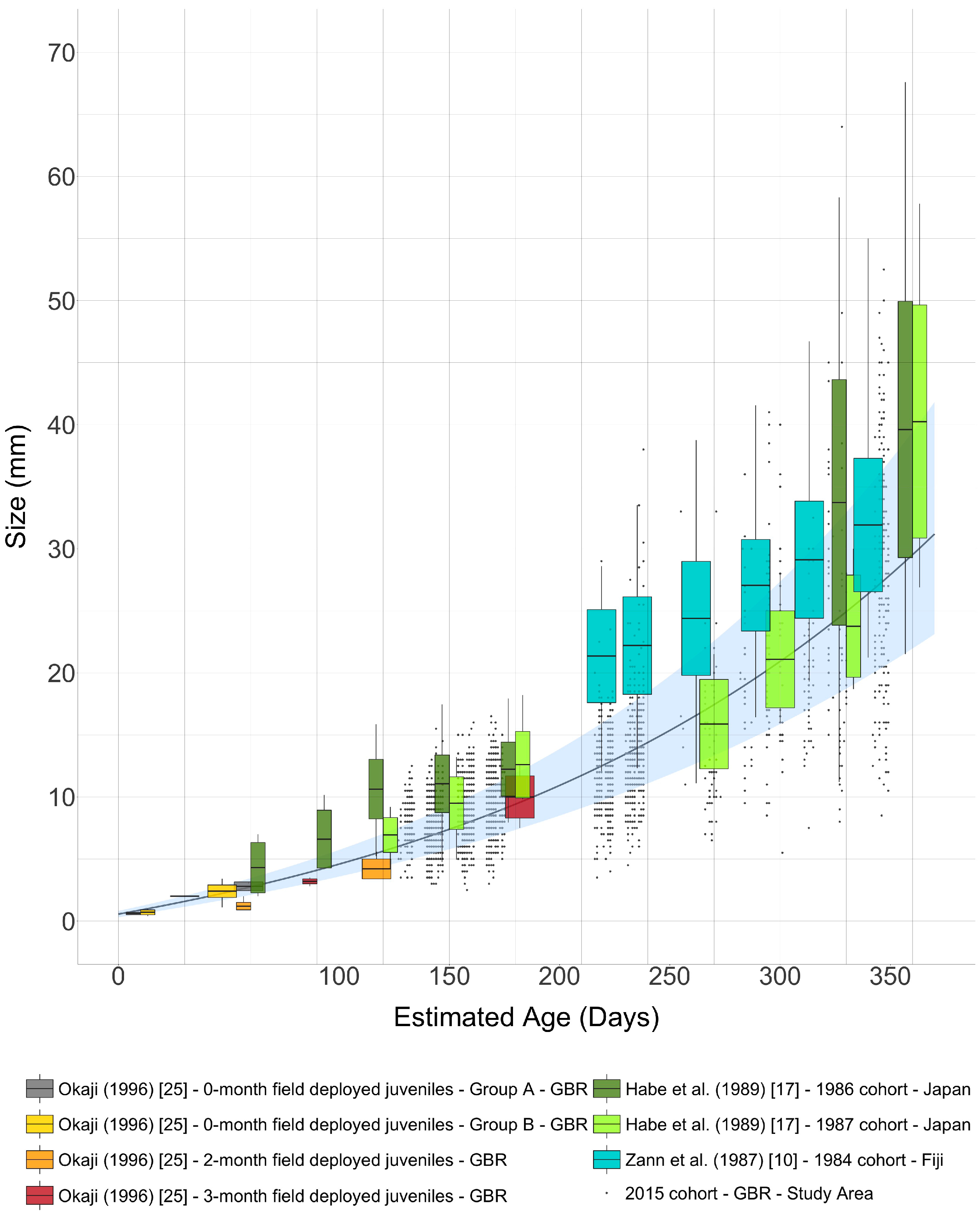
| Estimated Age after Metamorphosis (Months) | Time Span (30-Day Interval) | Range of Modelled Mean Sizes (mm) | Range of Modelled Growth Rates (mm·Day−1) | |
|---|---|---|---|---|
| 1 | 0–30 | 0.30–2.04 | 0.028–0.041 | |
| 2 | 30–60 | 1.15–3.46 | 0.032–0.047 | |
| 3 | 60–90 | 2.11–5.12 | 0.036–0.055 | |
| 4 | 90–120 | 3.19–7.04 | 0.041–0.064 | |
| 5 | 120–150 | 4.41–9.28 | 0.046–0.075 | |
| 6 | 150–180 | 5.79–11.89 | 0.052–0.087 | |
| 7 | 180–210 | 7.35–14.93 | 0.059–0.101 | |
| 8 | 210–240 | 9.11–18.47 | 0.066–0.118 | |
| 9 | 240–270 | 11.09–22.58 | 0.075–0.137 | |
| 10 | 270–300 | 13.33–27.37 | 0.084–0.160 | |
| 11 | 300–330 | 15.87–32.94 | 0.095–0.186 | |
| 12 | 330–360 | 18.73–39.43 | 0.108–0.216 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmes, J.; Matthews, S.; Schultz, D.; Messmer, V.; Hoey, A.; Pratchett, M. Modelling Growth of Juvenile Crown-of-Thorns Starfish on the Northern Great Barrier Reef. Diversity 2017, 9, 1. https://doi.org/10.3390/d9010001
Wilmes J, Matthews S, Schultz D, Messmer V, Hoey A, Pratchett M. Modelling Growth of Juvenile Crown-of-Thorns Starfish on the Northern Great Barrier Reef. Diversity. 2017; 9(1):1. https://doi.org/10.3390/d9010001
Chicago/Turabian StyleWilmes, Jennifer, Samuel Matthews, Daniel Schultz, Vanessa Messmer, Andrew Hoey, and Morgan Pratchett. 2017. "Modelling Growth of Juvenile Crown-of-Thorns Starfish on the Northern Great Barrier Reef" Diversity 9, no. 1: 1. https://doi.org/10.3390/d9010001






