Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. solaris) at Varying Algal Cell Densities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Larval Culture and Settlement Experiments
2.2. Statistical Analyses
3. Results
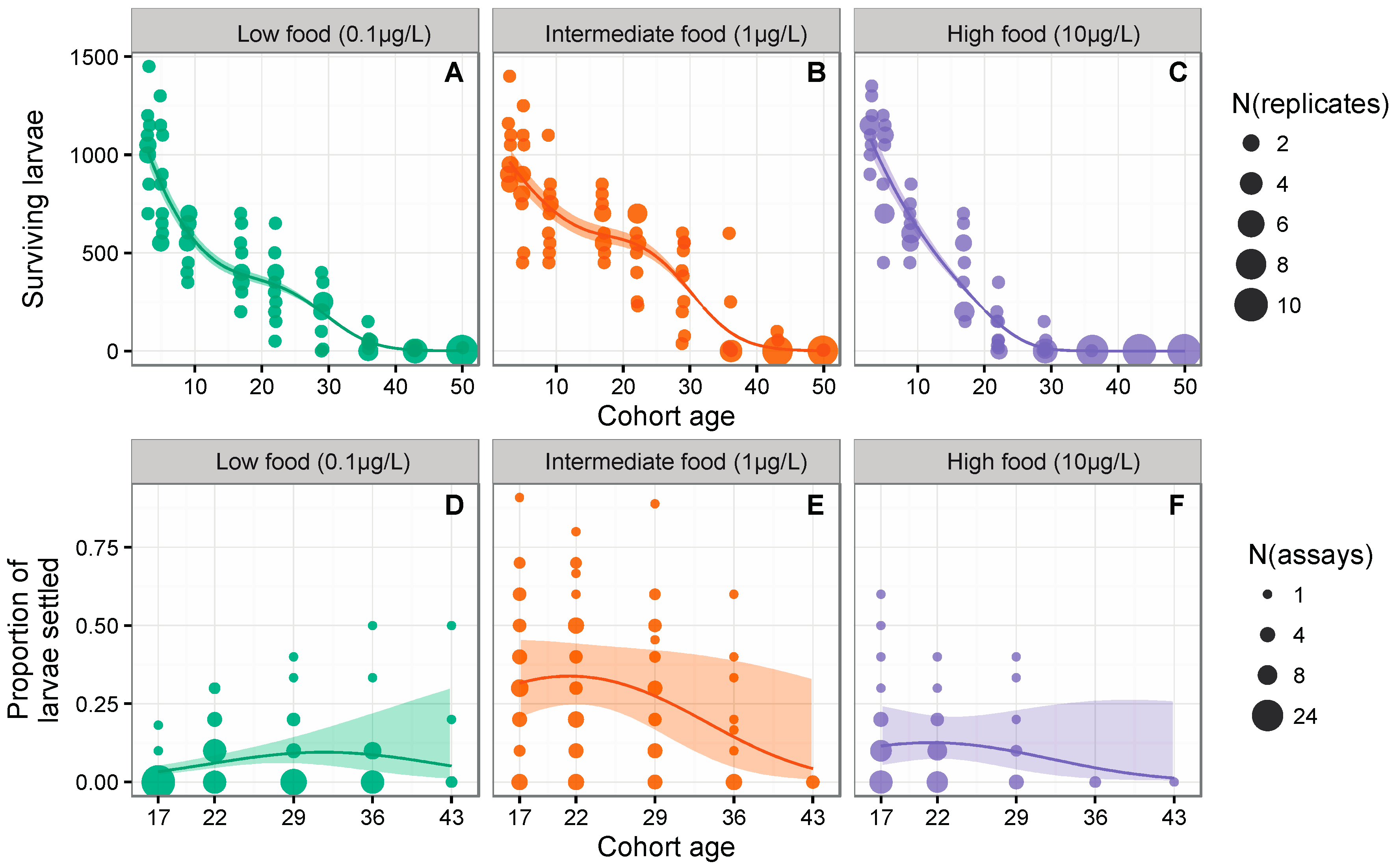
3.1. Larval Survival
3.2. Probability of Successful Settlement
4. Discussion
4.1. Non-Linear Effects of Increasing Food Availability on Larval Survivorship and Settlement
4.2. Minimum and Maximum Competency Periods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biol. Bull. 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Scheltema, R.S. Long-distance dispersal by planktonic larvae of shoal-water benthic invertebrates among central Pacific islands. Bull. Mar. Sci. 1986, 39, 241–256. [Google Scholar]
- Lawton, R.J.; Messmer, V.; Pratchett, M.S.; Bay, L.K. High gene flow across large geographic scales reduces extinction risk for a highly specialised coral feeding butterflyfish. Mol. Ecol. 2011, 20, 3584–3598. [Google Scholar] [CrossRef] [PubMed]
- Almany, G.R.; Berumen, M.L.; Thorrold, S.R.; Planes, S.; Jones, G.P. Local replenishment of coral reef fish populations in a marine reserve. Science 2007, 316, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Nanninga, G.B.; Berumen, M.L. The role of individual variation in marine larval dispersal. Front. Mar. Sci. 2014, 1, 71. [Google Scholar] [CrossRef]
- Pratchett, M.S. Dynamics of an outbreak population of Acanthaster planci at Lizard Island, northern Great Barrier Reef (1995–1999). Coral Reefs 2005, 24, 453–462. [Google Scholar] [CrossRef]
- Wooldridge, S.A.; Brodie, J.E. Environmental triggers for primary outbreaks of crown-of-thorns starfish on the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2015, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hock, K.; Wolff, N.H.; Condie, S.A.; Anthony, K.R.N.; Mumby, P.J. Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J. Appl. Ecol. 2014, 51, 1188–1196. [Google Scholar] [CrossRef]
- Victor, B.C. Settlement strategies and biogeography of reef fishes. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 231–260. [Google Scholar]
- Richmond, R.H. Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar. Biol. 1987, 93, 527–533. [Google Scholar] [CrossRef]
- Graham, E.M.; Baird, A.H.; Connolly, S.R. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 2008, 27, 529–539. [Google Scholar] [CrossRef]
- Henderson, J.A.; Lucas, J.S. Larval development and metamorphosis of Acanthaster planci (Asteroidea). Nature 1971, 232, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Lamare, M.D.; Pecorino, D.; Hardy, N.; Liddy, M.; Byrne, M.; Uthicke, S. The thermal tolerance of crown-of-thorns (Acanthaster planci) embryos and bipinnaria larvae: Implications for spatial and temporal variation in adult populations. Coral Reefs 2014, 33, 207–219. [Google Scholar] [CrossRef]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.S.; Hardy, N.; Lamare, M.D. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5, 8402. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.R. In situ culturing as a test of the larval starvation hypothesis for the crown-of-thorns starfish, Acanthaster planci. Limnol. Oceanogr. 1987, 32, 895–904. [Google Scholar] [CrossRef]
- Lucas, J.S. Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J. Exp. Mar. Biol. Ecol. 1982, 65, 173–193. [Google Scholar] [CrossRef]
- Brodie, J.E.; Fabricius, K.E.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Haszprunar, G.; Spies, M. An integrative approach to the taxonomy of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): A review of names and comparison to recent molecular data. Zootaxa 2014, 3841, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.Z.; Dworjanyn, S.A.; Hardy, N.; Mos, B.; Uthicke, S.; Byrne, M. Larvae of the coral eating crown-of-thorns starfish, Acanthaster planci in a warmer-high CO2 ocean. Glob. Chang. Biol. 2014, 20, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Okaji, K.; Ayukai, T.; Lucas, J.S. Selective feeding by larvae of the crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1997, 16, 47–50. [Google Scholar] [CrossRef]
- Mellin, C.; Lugrin, C.; Okaji, K.; Francis, D.S.; Uthicke, S. Selective feeding and microalgal consumption rates by crown-of-thorns seastar (Acanthaster cf. solaris) larvae. Diversity 2016. under review. [Google Scholar]
- Cowan, Z.L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Rumrill, S.S. Natural mortality of marine invertebrate larvae. Ophelia 1990, 32, 163–198. [Google Scholar] [CrossRef]
- Mos, B.; Cowden, K.L.; Nielsen, S.J.; Dworjanyn, S.A. Do cues matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PLoS ONE 2011, 6, e28054. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wood, S.N.; Scheipl, F. Gamm4: Generalized Additive Mixed Models Using mgcv and lme4. R Packag Version 0.2-3. Available online: http://CRAN.R-project.org/package=gamm4 (accessed on 5 May 2016).
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Skaug, H.J.; Fournier, D.A.; Nielsen, A.; Magnusson, A.; Bolker, B.M. Generalized Linear Mixed Models using AD Model Builder. R Package version 0.8.3.3. Available online: http://glmmadmb.r-forge.r-project.org (accessed on 19 January 2016).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 21 June 2016).
- Caballes, C.F.; Pratchett, M.S.; Kerr, A.M.; Rivera-Posada, J.A. The role of maternal nutrition on oocyte size and quality, with respect to early larval development in the coral-eating starfish, Acanthaster planci. PLoS ONE 2016, 11, e0158007. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O. Uptake of dissolved organic matter by larval stage of the crown-of-thorns starfish Acanthaster planci. Mar. Biol. 1994, 120, 55–63. [Google Scholar]
- Nakajima, R.; Nakatomi, N.; Kurihara, H.; Fox, M.; Smith, J.; Okaji, K. Crown-of-thorns starfish larvae can feed on organic matter released from corals. Diversity 2016, 8, 18. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves: A Practical Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Brodie, J.E.; De’ath, G.; Devlin, M.J.; Furnas, M.J.; Wright, M. Spatial and temporal patterns of near-surface chlorophyll a in the Great Barrier Reef lagoon. Mar. Freshw. Res. 2007, 58, 342–353. [Google Scholar] [CrossRef]
- Wolanski, E.; Jones, M.; Williams, W.T.; Wolanski, E.; Jones, M.; Williams, W. Physical properties of the Great Barrier reef Lagoon waters near Townsville. II. Seasonal variations. Mar. Freshw. Res. 1981, 32, 321–334. [Google Scholar] [CrossRef]
- Brodie, J.E.; Schroeder, T.; Rohde, K.; Faithful, J.; Masters, B.; Dekker, A.G.; Brando, V.E. Dispersal of suspended sediments and nutrients in the Great Barrier Reef lagoon during river discharge events: Conclusions from satellite remote sensing and concurrent flood plume sampling. Brodie Jon. Mar. Freshw. Res. 2010, 61, 651–664. [Google Scholar] [CrossRef]
- Babcock, R.C.; Milton, D.A.; Pratchett, M.S. Relationships between size and reproductive output in the crown-of-thorns starfish. Mar. Biol. 2016, 163, 234. [Google Scholar] [CrossRef]
- Luiz, O.J.; Allen, A.P.; Robertson, D.R.; Floeter, S.R.; Kulbicki, M.; Vigliola, L.; Becheler, R.; Madin, J.S. Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc. Natl. Acad. Sci. USA 2013, 110, 16498–16502. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, E.; Spagnol, S. Sticky waters in the Great Barrier Reef. Estuar. Coast. Shelf Sci. 2000, 50, 27–32. [Google Scholar] [CrossRef]
- Lucas, J.S. Environmental influences on the early development of Acanthaster planci (L.). In Crown-of-Thorns Starfish Seminar Proceedings; Austrlian Government Publishing Service: Canberra, Australia, 1974; pp. 109–121. [Google Scholar]
- Strathmann, R.R. Length of pelagic period in echinoderms with feeding larvae from the Northeast Pacific. J. Exp. Mar. Bio. Ecol. 1978, 34, 23–27. [Google Scholar] [CrossRef]
- Kenchington, R.A. Growth and recruitment of Acanthaster planci (L.) on the Great Barrier Reef. Biol. Conserv. 1977, 11, 103–118. [Google Scholar] [CrossRef]
- Vanhatalo, J.; Hosack, G.R.; Sweatman, H.P.A. Spatio-temporal modelling of crown-of-thorns starfish outbreaks on the Great Barrier Reef to inform control strategies. J. Appl. Ecol. 2016. [Google Scholar] [CrossRef]
- Yasuda, N.; Nagai, S.; Hamaguchi, M.; Okaji, K.; Gérard, K.; Nadaoka, K. Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Mol. Ecol. 2009, 18, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Timmers, M.A.; Bird, C.E.; Skillings, D.J.; Smouse, P.E.; Toonen, R.J. There’s no place like home: Crown-of-thorns outbreaks in the central pacific are regionally derived and independent events. PLoS ONE 2012, 7, e31159. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Benzie, J.A.H.; Lessios, H.A.; Barber, P.H.; Wörheide, G. A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol. Lett. 2008, 4, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Haszprunar, G.; Vogler, C.; Wörheide, G. Taxonomy does matter—A plea for the use of DNA-barcoding and barcode identification numbers when studying crown-of-thorns seastars (Acanthaster planci species complex). Diversity 2016. under review. [Google Scholar]
- Timmers, M.A.; Andrews, K.R.; Bird, C.E.; DeMaintenton, M.J.; Brainard, R.E.; Toonen, R.J. Widespread dispersal of the crown-of-thorns sea Star, Acanthaster planci, across the Hawaiian Archipelago and Johnston Atoll. J. Mar. Biol. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Yasuda, N.; Taquet, C.; Nagai, S.; Yoshida, T.; Adjeroud, M. Genetic connectivity of the coral-eating sea star Acanthaster planci during the severe outbreak of 2006–2009 in the Society Islands, French Polynesia. Mar. Ecol. 2015, 36, 668–678. [Google Scholar] [CrossRef]
- Harrison, H.B.; Pratchett, M.S.; Messmer, V.; Saenz-Agudelo, P.; Berumen, M.L. Microsatellites reveal genetic homogeneity among outbreak populations of crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 2016. under review. [Google Scholar]
| (a) Parametric Coefficients | Estimate | Std. Error | p | |
| Intercept | 4.59 | 0.05 | <2 × 10−16 | |
| treatment (medium) | 0.51 | 0.04 | <2 × 10−16 | |
| treatment (high) | −3.50 | 0.23 | <2 × 10−16 | |
| (b) Smooth Terms | edf | Ref.df | Chi.sq | p |
| s(time) treatment (low) | 2.997 | 3 | 12,436 | <2 × 10−16 |
| s(time) treatment (medium) | 2.998 | 3 | 9927 | <2 × 10−16 |
| s(time) treatment (high) | 2.985 | 3 | 10,680 | <2 × 10−16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratchett, M.S.; Dworjanyn, S.; Mos, B.; Caballes, C.F.; Thompson, C.A.; Blowes, S. Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. solaris) at Varying Algal Cell Densities. Diversity 2017, 9, 2. https://doi.org/10.3390/d9010002
Pratchett MS, Dworjanyn S, Mos B, Caballes CF, Thompson CA, Blowes S. Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. solaris) at Varying Algal Cell Densities. Diversity. 2017; 9(1):2. https://doi.org/10.3390/d9010002
Chicago/Turabian StylePratchett, Morgan S., Symon Dworjanyn, Benjamin Mos, Ciemon F. Caballes, Cassandra A. Thompson, and Shane Blowes. 2017. "Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. solaris) at Varying Algal Cell Densities" Diversity 9, no. 1: 2. https://doi.org/10.3390/d9010002







