Evaluation of Protein Adsorption on Chitosan Surfaces with Reflectometry Interference Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1 RIfS Apparatus
2.2 Preparation of Chitosan and PS films
2.3. Proteins
2.4 Protein Adsorption Measurement
2.5 Calculation of the Adsorbed Layer of Protein

 , the average thickness of each type of proteins on the surface of the material intended to be probed with RIfS.
, the average thickness of each type of proteins on the surface of the material intended to be probed with RIfS.2.6 AFM Imaging
3. Results and Discussion
3.1 Kinetics of Protein Adsorption onto PS Films
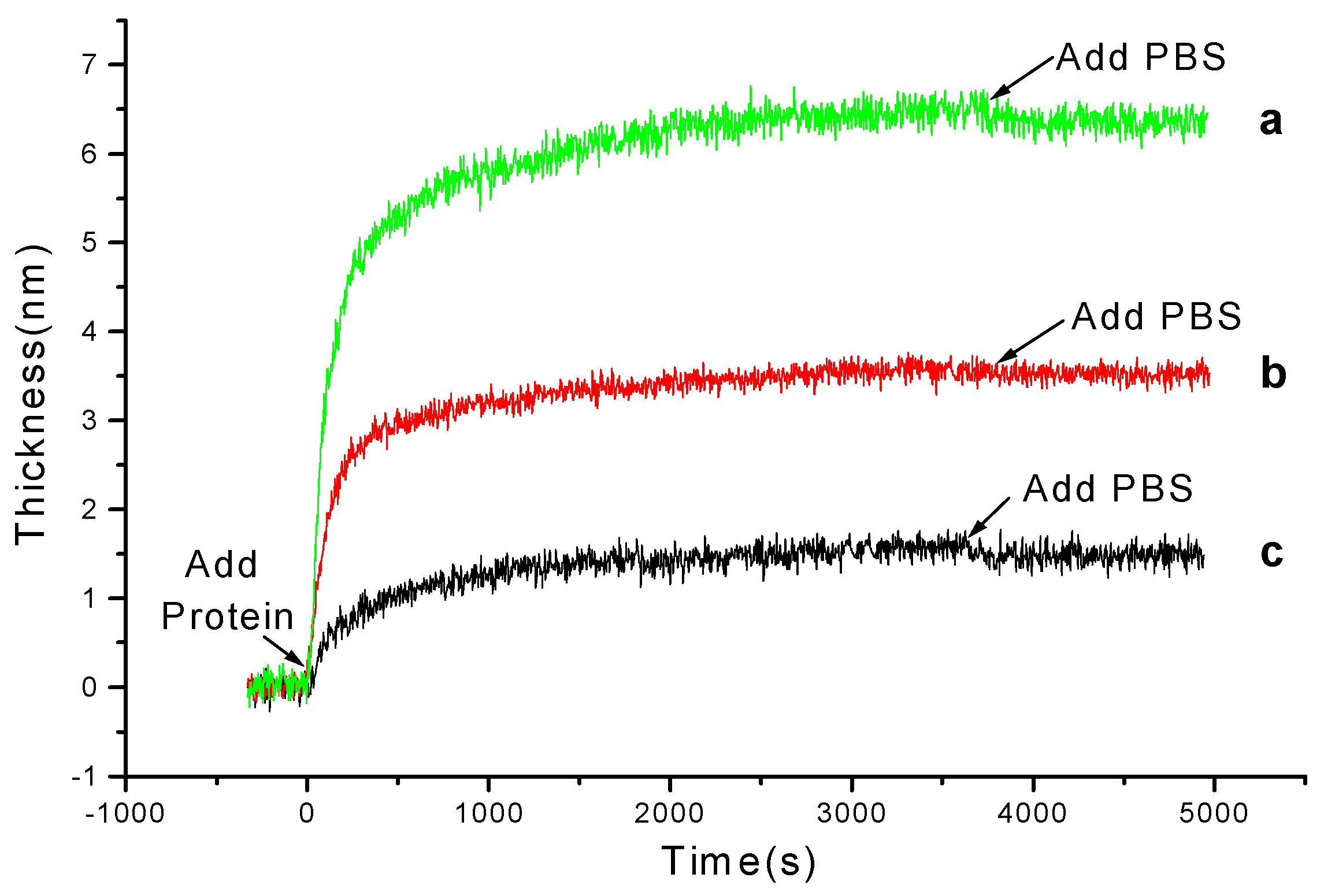
3.2 Kinetics of Protein Adsorption onto Chitosan Films
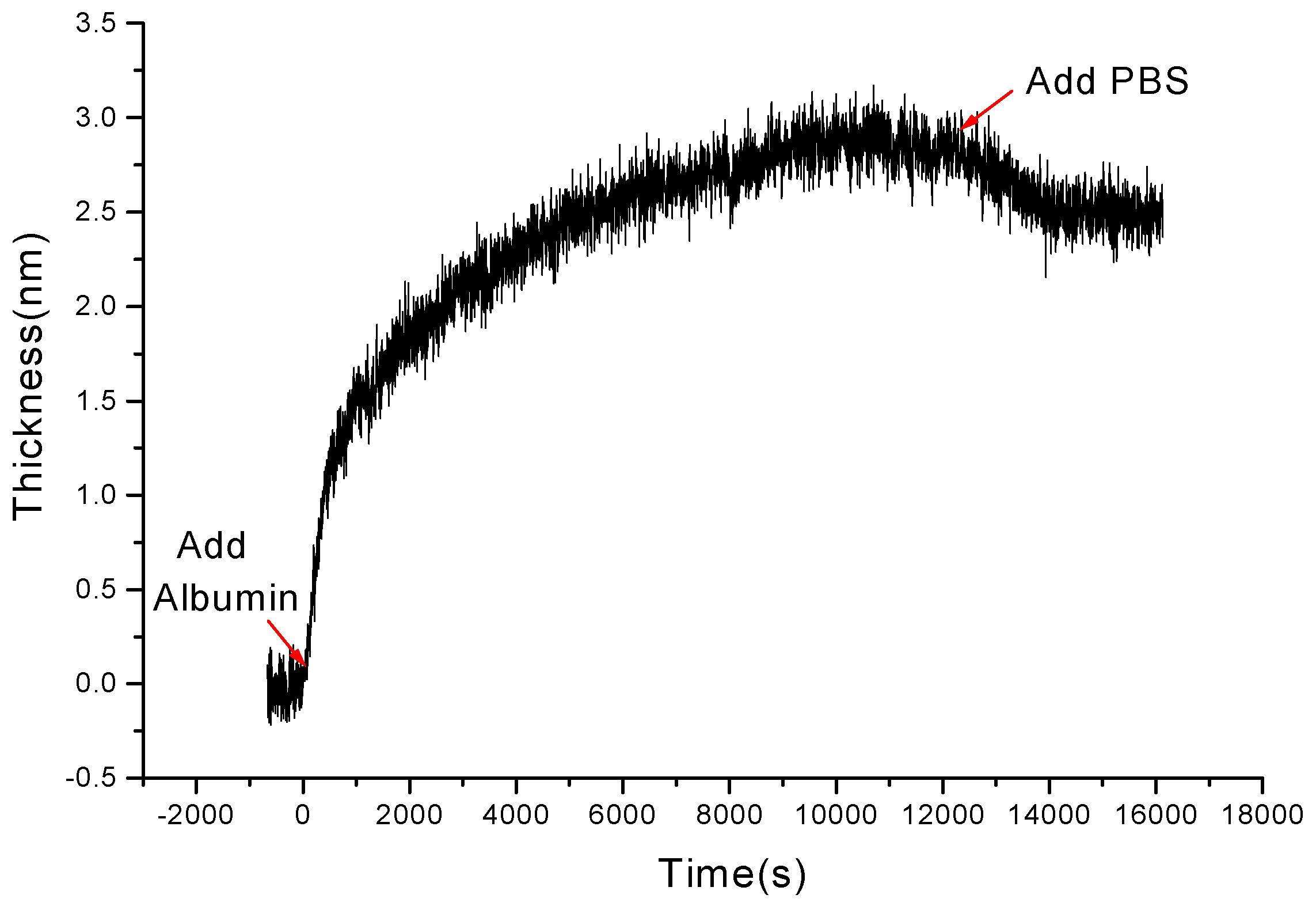
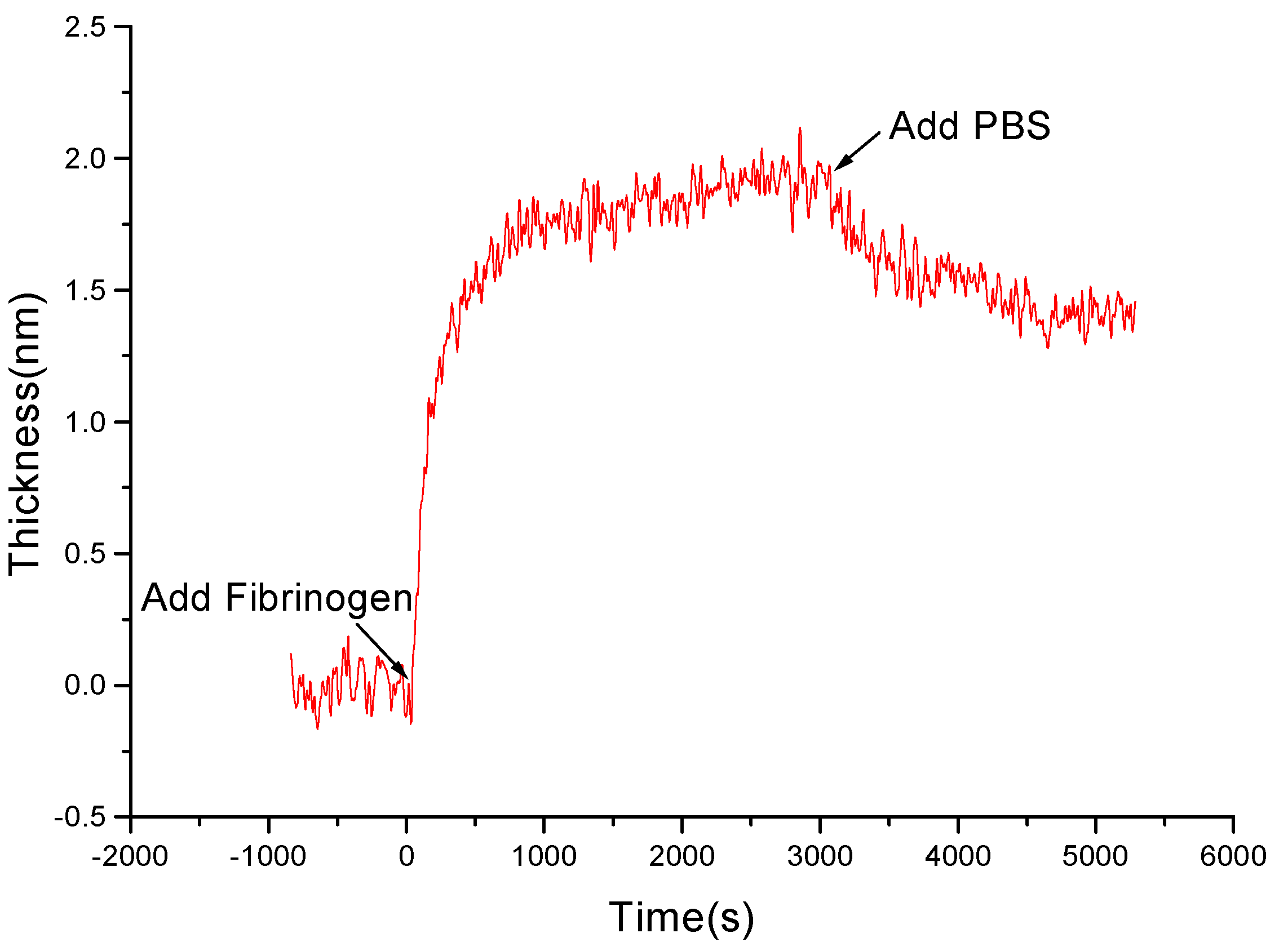

3.3 Comparing of the Kinetic Curves on Chitosan and PS

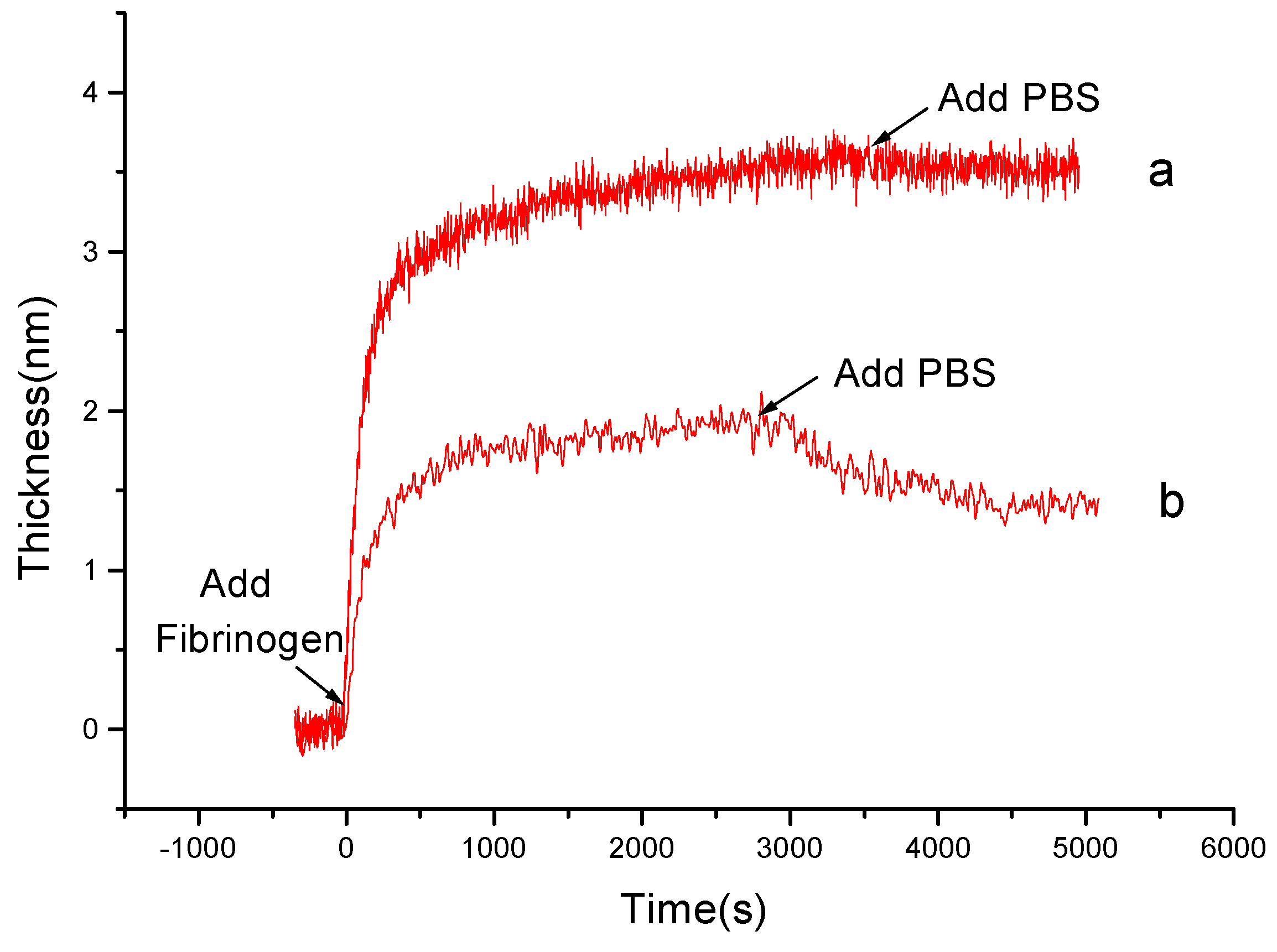

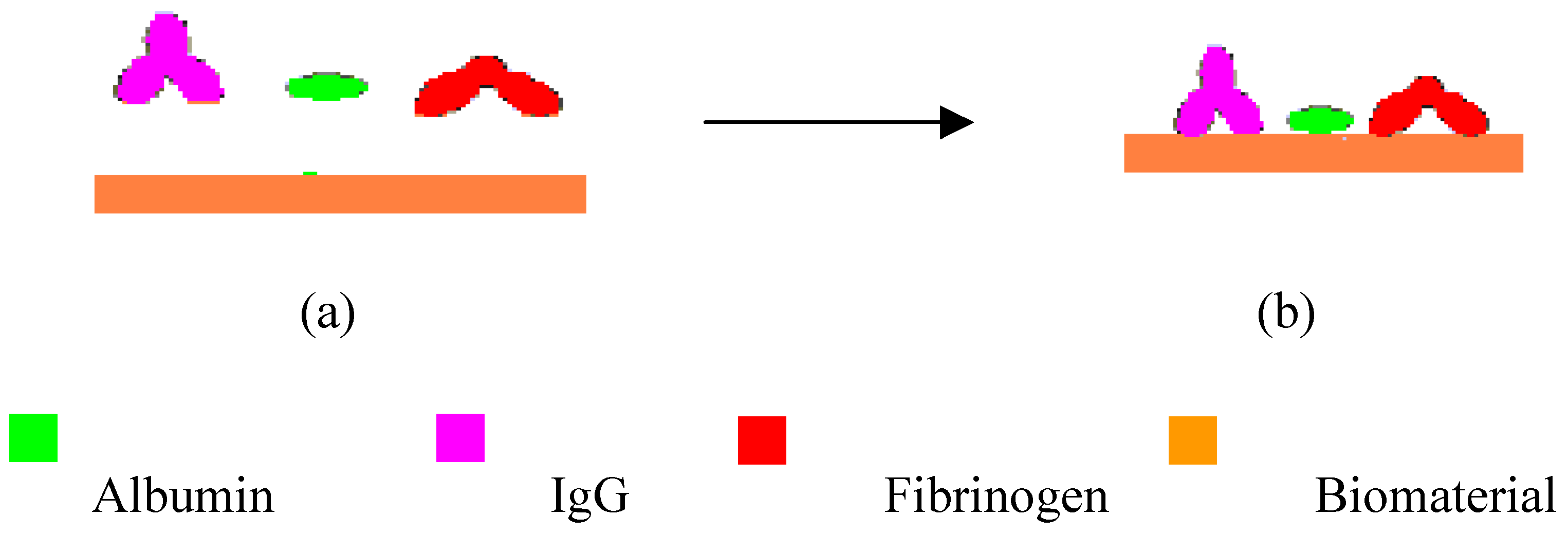
3.4 Adsorbed Layers of Alb, Fib, and IgG on Chitosan and PS Films
| Thickness Proteins | (n=3) | SD | Adsorbed Layer |
|---|---|---|---|
| Alb | 1.34 | 0.0868 | 0.336 |
| Fib | 3.59 | 0.0586 | 0.399 |
| IgG | 6.35 | 0.0430 | 0.577 |
| Thickness Proteins | (n=3) | SD | Adsorbed Layer |
|---|---|---|---|
| Alb | 2.54 | 0.0193 | 0.635 |
| Fib | 1.42 | 0.0220 | 0.158 |
| IgG. | 1.06 | 0.0505 | 0.0967 |
3.5 AFM characteristic
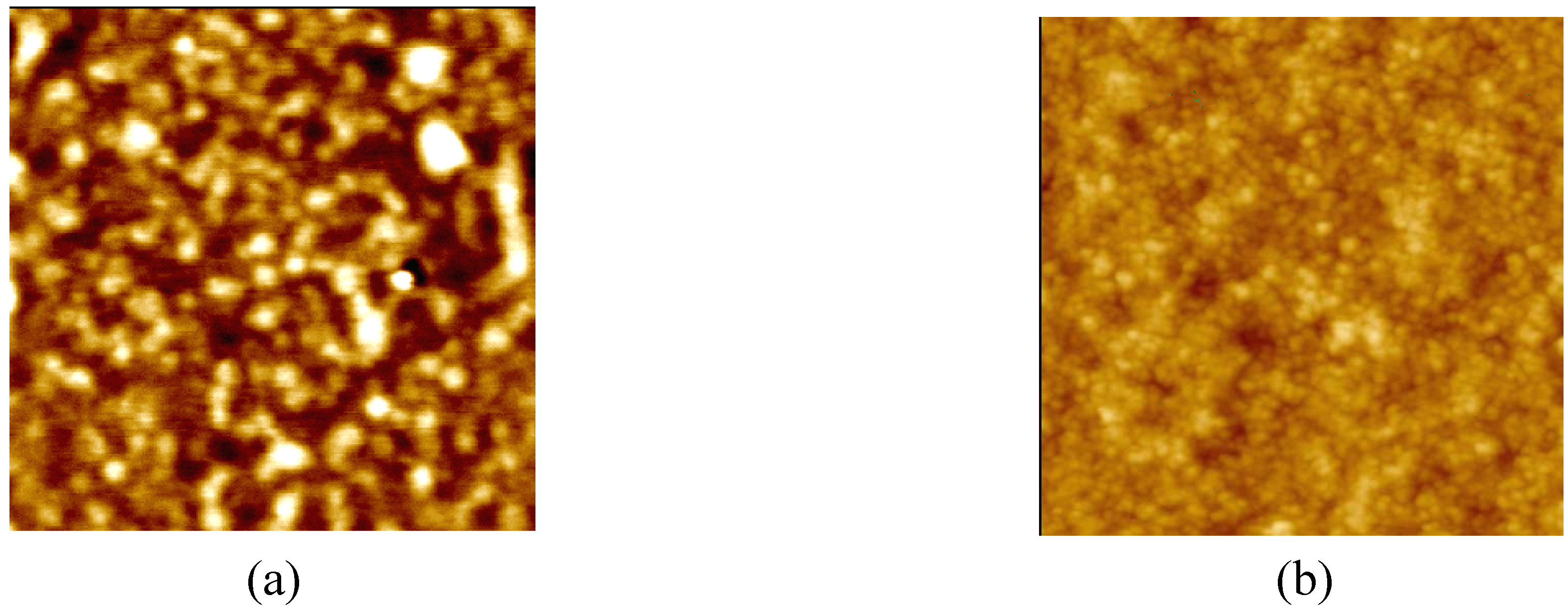
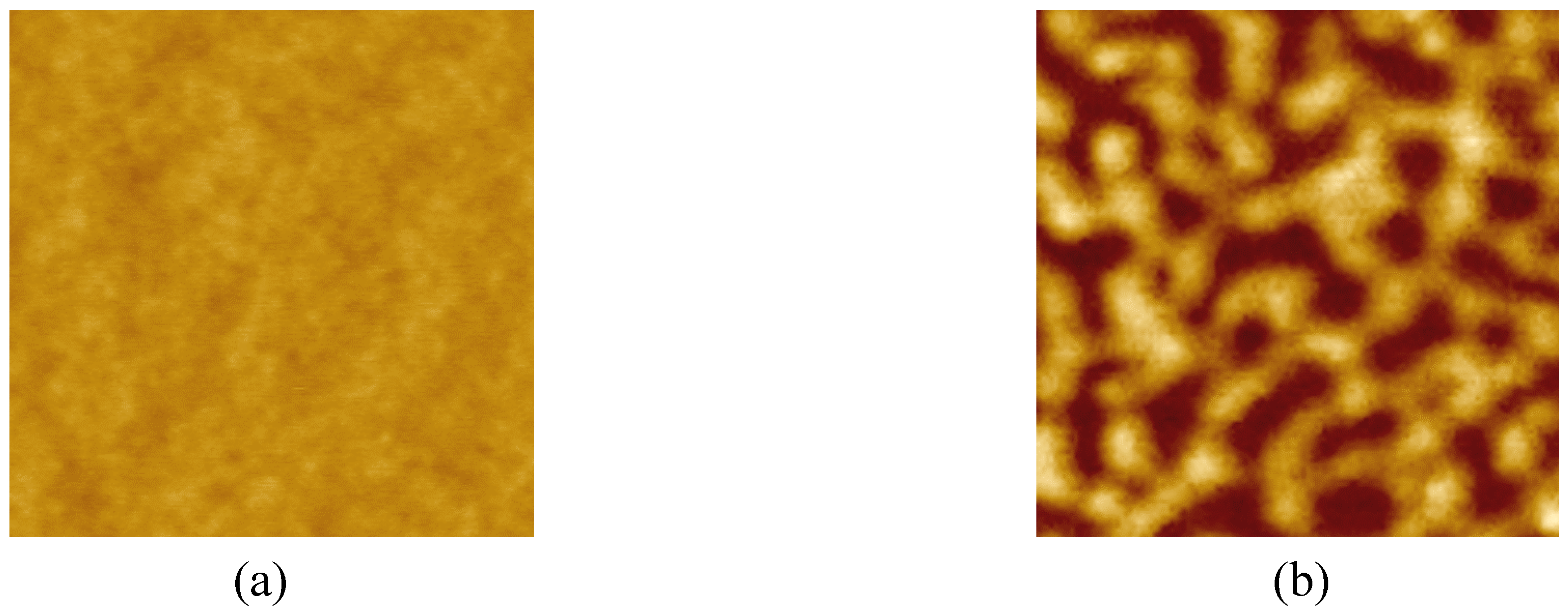
4. Conclusions
Acknowledgements
References
- Kawaguchi, T.; Shiro, T.; Iwata, K. A highly sensitive device for visual detection of antigens and antibodies by means of light interference. Sensors and Actuators B 1991, 3, 113–121. [Google Scholar] [CrossRef]
- Brecht, A.; Gauglitz, G.; Nahm, W. Interferometric measurement used in chemical and biochemical sensors. Analysis 1992, 20, 135–140. [Google Scholar]
- Myszka, D.G. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Current Opinion in Biotechnology 1997, 8, 50–57. [Google Scholar] [CrossRef]
- Green, R.J.; Frazier, R.A.; Shakesheff, K.M.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B. Surface plasmon resonance analysis of dynamic biological interactions with biomaterials. Biomaterials 2000, 21, 1823–1835. [Google Scholar] [CrossRef]
- Brecht, A.; Ingenhoff, J.; Gauglitz, G. Direct monitoring of antigen-antibody interactions by spectral interferometry. Sensors and Actuators B 1992, 6, 96–100. [Google Scholar] [CrossRef]
- Gauglitz, G.; Brecht, A.; Kraus, G.; Nahm, W. Chemical and biochemical sensors based on interferometry at thin (multi-) layers. Sensors and Actuators B 1993, 11, 21–27. [Google Scholar] [CrossRef]
- Schmitt, H.M.; Brecht, A.; Piehler, J.; Gauglitz, G. An integrated system for optical biomolecular interaction analysis. Biosensors & Bioelectronics 1997, 12(8), 809–816. [Google Scholar]
- Sauer, M.; Brecht, A.; Charissé, K.; Maier, M.; Gerster, M.; Stemmler, I.; Gauglitz, G.; Bayer, E. Interaction of Chemically Modified antisense oligonucleotides with sense DNA: a label-free interaction study with reflectometric interference spectroscopy. Analytical Chemistry 1999, 71, 2850–2857. [Google Scholar] [CrossRef]
- Brecht, A.; Gauglitz, G. Optimised layer systems for immunosensors used on the RIFS transducer. J Anal Chem. 1994, 349, 360–366. [Google Scholar] [CrossRef]
- Piehler, J.; Brecht, A.; Gauglitz, G.; Maul, C.; Grabley, S.; Zerlin, M. Specific binding of lowmolecular weight ligands with direct optical detection. Biosensors & Bioelectronics 1997, 12(6), 531–538. [Google Scholar] [CrossRef]
- Rathgeb, F.; Gauglitz, G. Dyeless optical detection of ammonia in the gas phase using pH- responsive polymers with reflectometric interference spectroscopy. Analytica Chimica Acta 1998, 372, 333–340. [Google Scholar] [CrossRef]
- Yu, F.; Yao, D.F.; Qian, W.P.; et al. Reflectometry. interference spectroscopy in detection of hepatitis B surface antigen. Clin. Chem. 2000, 46(9), 1489–1490. [Google Scholar]
- Lü, X.Y.; Huang, H.F.; Chen, D.M.; et al. Real time in situ kinetic analysis of proteins adsorbed onto the surface of hydroxyapatite film using RIfS. In Proceedings of the first international conference on biomaterials (China), Beijing, China, July 24–26,2001.
- Sharma, C.P.; Sunny, M.C. Albumin adsorption on to aluminum oxide and polyurethane surfaces. Biomaterials 1990, 11, 255–257. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, D.J.; Zhao, J.; Gu, H.Q. A study of blood protein adsorption on diamond-like carbon. In Chinese Conference on Biomaterials, Panyu. China; November 1999; pp. 5–8. [Google Scholar]
- Babensee, J.E.; Cornelius, R.M.; Brash, J.L.; Sefton, M.V. Immunoblot analysis of proteins associated with HEMA-MMA microcapsules: Human serum proteins in vitro and rat proteins following implantation. Biomaterials 1998, 19, 839–849. [Google Scholar] [CrossRef]
- Kandori, K.; Fujiwara, A.; Mukai, M.; Yasukawa, A.; Ishikawa, T. Evaluation of the adsorption affinity of proteins to calcium hydroxyapatites by desorption and pre-adsorption methods. Colloids and Surfaces B: Biointerfaces 1998, 11, 313–320. [Google Scholar] [CrossRef]
- Klomp, A.J.A.; Engbers, G.H.M.; Mol, J.; Terlingen, J.G.A.; Feijen, J. Adsorption of proteins from plasma at polyester non-wovens. Biomaterials 1999, 20, 1203–1211. [Google Scholar] [CrossRef]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar]
- Zeng, H.; Chittur, K.K.; Lacefield, W.R. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials 1999, 20, 377–384. [Google Scholar] [CrossRef]
- Kingshott, P.; Heather, A.W.; John, S.T.; Griesser, H.J. Direct detection of proteins adsorbed on synthetic materials by matrix-assisted laser desorption ionization-mass spectrometry. Analytical Biochemistry 1999, 273, 156–162. [Google Scholar] [CrossRef]
- Oleschuk, R.D.; McComb, M.E.; Chow, A.; Ens, W.; Standing, K.G.; Perresult, H.; Mariois, Y.; King, M. Characterization of plasma proteins adsorbed onto biomaterials by MALDI-TOFMS. Biomaterials 2000, 21, 1701–1710. [Google Scholar] [CrossRef]
- Ortega-Vinuesa, J.L.; Tengvall, P.; Wälivaara, B.; Lundström, I. Stagnant versus dynamic conditions: a comparative adsorption study of blood proteins. Biomaterials 1998, 19, 251–262. [Google Scholar] [CrossRef]
- Zhang, M.; Desai, T.; Ferrari, M. Proteins and cells on PEG immobilized silicon surfaces. Biomaterials 1998, 19, 953–960. [Google Scholar] [CrossRef]
- Elwing, H. Protein absorption and ellipsometry in biomaterial research. Biomaterials 1998, 19, 397–406. [Google Scholar] [CrossRef]
- Tengyall, P.; Lundström, I.; Liedberg, B. Protein adsorption studies on model organic surfaces: an ellipsometric and infrared spectroscopic approach. Biomaterials 1998, 19, 407–422. [Google Scholar] [CrossRef]
- Hirano, S.; Zhang, M.; Nakagawa, M.; Miyata, T. Wet spun chitosan-collagen fibers, their chemical N-modifications, and blood compatibility. Biomaterials 2000, 21, 997–1003. [Google Scholar] [CrossRef]
- Gu, H.Q.; Xu, G.F. Biomedical Materials. Publishing Company. Of Translate for Sci and Tech.: Tianjing, China, 1993; pp. 316–324. [Google Scholar]
- Zhang, J.X.; Tang, J.; Xu, B. Biocompatibility and safety evaluation of chitosan rod. J Biomed Eng. (Chinese) 1996, 13(4), 293–297. [Google Scholar]
- Jiang, X.S.; Wang, B.S.; Chen, C.; Li, X.G.; Sun, F.Y. The bioactivity and medical appliance of chitin and its ramification. J Biomed Eng. (Chinese) 1996, 13(4), 353–356. [Google Scholar]
- Andrew, C.A.W.; Khor, E.; Hastings, G.W. The influence of anionic chitin derivatives on calcium phosphate crystallization. Biomaterials 1998, 19, 1309–1316. [Google Scholar] [CrossRef]
- Leroux, L.; Freche, Z.; Frèche, M.; Lacout, J.L. Effects of various adjuvants (lactic acid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone 1999, 25(2), 318–348. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Campos, A.M.D.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. International Journal of Pharmaceutics 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; Van Aelst, A.C.; Borchard, G.; Junginger, H.E. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 2001, 22(7), 687–694. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Chen, S.Q. The mechanism of chitosan hemostasis and its application. Alien Medical: Biomedical and engineering 2001, 24(2), 77–80. [Google Scholar]
- Amiji, M.M. Platelet adhension and activation on an amphoteric chitosan derivative bearing sulfonate groups. Colloids and Surfaces B:Biointerfaces 1998, 10, 263–271. [Google Scholar] [CrossRef]
- Lin, C.W.; Lin, J.C. Surface characterization and platelet compatibility evalution of surface- sulfonated chitosan membrane. J Biomater Sci Polym Ed. 2001, 12(5), 543–557. [Google Scholar] [CrossRef]
- Green, R.J.; Davies, J.; Davies, M.C.; Robert, C.J.; Tendler, S.J.B. Surface plasmon resonance for real time in situ analysis of protein adsorption to polymer surfaces. Biomaterials 1997, 18, 405–413. [Google Scholar] [CrossRef]
- Yu, F. The establishment of reflectometry interference spectroscopy and its use in the token of interface biomolecule. M.S.Thesis, Biomedical and Engineering Department of Southeast University, Nanjing, China, July 2000. [Google Scholar]
- Huang, S.L.; Chao, M.S.; Ruan, R.C.; Lai, J.Y. Microphase separated structure and protein adsorption of polyurethanes with butadiene soft segment. European Polymer Journal 2000, 36, 285–294. [Google Scholar] [CrossRef]
- Qian, W.P.; Yao, D.F.; Yu, F.; Xu, B.; Zhou, R.; Bao, X.; Lu, Z.H. Immobilization of antibodies on ultraflat polystyrene surfaces. In AACC’s Oak Ridge Conference, Bosten, USA, 2000 May; pp. 5–6.
- Sample Availability: Available from the author.
© 2001 by MDPI (http://www.mdpi.net) Reproduction is permitted for noncommercial purposes.
Share and Cite
Lü, X.Y.; Huang, Y.; Ma, C.Q. Evaluation of Protein Adsorption on Chitosan Surfaces with Reflectometry Interference Spectroscopy. Sensors 2001, 1, 148-160. https://doi.org/10.3390/s10500148
Lü XY, Huang Y, Ma CQ. Evaluation of Protein Adsorption on Chitosan Surfaces with Reflectometry Interference Spectroscopy. Sensors. 2001; 1(5):148-160. https://doi.org/10.3390/s10500148
Chicago/Turabian StyleLü, Xiao Ying, Yan Huang, and Chao Qun Ma. 2001. "Evaluation of Protein Adsorption on Chitosan Surfaces with Reflectometry Interference Spectroscopy" Sensors 1, no. 5: 148-160. https://doi.org/10.3390/s10500148




