Formation of Zwitterionic Fullerodendron Using a New DBN-Focal Dendron
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
Preparation of dendron 1a
Preparation of dendron 1b
Formation of zwitterionic fullerodendrons 9 or 12
Preparation of zwitterionic fullerodendron 12a
4. Conclusions
Acknowledgments
References and Notes
- Nierengarten, J.F. Topics in Current Chemistry; Schalley, C.A., Vögtle, F., Eds.; Springer: New York, NY, USA, 2003; Volume 228, pp. 87–110. [Google Scholar]
- Rio, Y.; Accorsi, G.; Nierengarten, H.; Bourgogne, C.; Strub, J.M.; van Dorsselaer, A.; Armaroli, N.; Nierengarten, J.F. A fullerene Core to Probe Dendritic Shielding Effects. Tetrahedron 2003, 59, 3833–3844. [Google Scholar]
- Nierengarten, J.F.; Armaroli, N.; Accorsi, G.; Rio, Y.; Eckert, J.F. [60] Fullerene: A Versatile Photoactive Core for Dendrimer Chemistry. Chem. Eur. J 2003, 9, 36–41. [Google Scholar]
- Takaguchi, Y.; Tajima, T.; Ohta, K.; Motoyoshiya, J.; Aoyama, H.; Wakahara, T.; Akasaka, T.; Fujitsuka, M.; Ito, O. Reversible Binding of C60 to an Anthracene Bearing a Dendritic Poly(amidoamine) Substituent to Give a Water-soluble Fullerodendrimer. Angew. Chem. Int. Ed 2002, 41, 817–819. [Google Scholar]
- Skiebe, A.; Hirsch, A.; Klos, H.; Gotschy, B. [DBU]C60. Spin Pairing in a Fullerene Salt. Chem. Phys. Lett 1994, 220, 138–140. [Google Scholar]
- Nagata, K.; Dejima, E.; Kikuchi, Y.; Hashiguchi, M. Kilogram-scale [60] Fullerene Separation from a Fullerene Mixture: Selective Complexation of Fullerenes with 1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU). Chem. Lett 2005, 34, 178–179. [Google Scholar]
- Takaguchi, Y.; Sako, Y.; Yanagimoto, Y.; Tsuboi, S.; Motoyoshiya, J.; Aoyama, H.; Wakahara, T.; Akasaka, T. Facile and Reversible Synthesis of an Acidic Water-soluble Poly (amidoamine) Fullerodendrimer. Tetrahedron Lett 2003, 44, 5777–5780. [Google Scholar]
- Takaguchi, Y.; Katayose, Y.; Yanagimoto, Y.; Motoyoshiya, J.; Aoyama, H.; Wakahara, T.; Maeda, Y.; Akasaka, T. Photoinduced Dithiolation of Fullerene [60] with Dendrimer Disulfide. Chem. Lett 2003, 32, 1124–1125. [Google Scholar]
- Hirano, C.; Imae, T.; Fujima, S.; Yanagimoto, Y.; Takaguchi, Y. Fabrication and Properties of Fullerodendron Thin Films. Langmuir 2005, 21, 272–279. [Google Scholar]
- Sandanayaka, A.S.D.; Zhang, H.; Takaguchi, Y.; Sako, Y.; Tamura, M.; Araki, Y.; Ito, O. Photoinduced Charge Separation and Charge Recombination of Fullerene Bearing Dendritic Poly (amidoamine) with Carboxylates at the Terminal in Aqueous Media. Chem. Commun 2005, 7, 5160–5162. [Google Scholar]
- Araki, Y.; Kunieda, R.; Fujitsuka, M.; Ito, O.; Motoyoshiya, J.; Aoyama, H.; Takaguchi, Y. Photoinduced Intermolecular Electron Transfer and Energy Transfer of C60 Dendrimers. C. R. Chimie 2006, 9, 1014–1021. [Google Scholar]
- Takaguchi, Y.; Yanagimoto, Y.; Fujima, S.; Tsuboi, S. Photooxygenation of Olefins, Phenol, and Sulfide Using Fullerodendrimer as Catalyst. Chem. Lett 2004, 33, 1142–1143. [Google Scholar]
- Talukdar, B.; Takaguchi, Y.; Yanagimoto, Y.; Tsuboi, S.; Ichihara, M.; Ohta, K. Fabrication and Photocatalytic Activity of Fullerodendron/CaCO3 Composites. Bull. Chem. Soc. Jpn 2006, 79, 1983–1987. [Google Scholar]
- Kawasaki, N.; Nagano, T.; Kubozono, Y.; Sako, Y.; Morimoto, Y.; Takaguchi, Y.; Fujiwara, A.; Chu, C.C.; Imae, T. Transport Properties of Field-effect Transistor with Langmuir-Blodgett Films of C60 Dendrimer and Estimation of Impurity Levels. Appl. Phys. Lett 2007, 91, 24351/1–24351/5. [Google Scholar]
- Kusai, H.; Nagano, T.; Imai, K.; Kubozono, Y.; Sako, Y.; Takaguchi, Y.; Fujiwara, A.; Akima, N.; Iwasa, Y.; Hino, S. Fabrication of Field-effect Transistor Devices with Fullerodendron by Solution Process. Appl. Phys. Lett 2006, 88, 173509/1–173509/3. [Google Scholar]
- Takaguchi, Y.; Tamura, M.; Sako, Y.; Yanagimoto, Y.; Tsuboi, S.; Uchida, T.; Shimamura, K.; Kimura, S.; Wakahara, T.; Maeda, Y.; Akasaka, T. Fullerodendron-assisted Dispersion of Single-walled Carbon Nanotubes via Noncovalent Functionalization. Chem. Lett 2005, 34, 1608–1609. [Google Scholar]
- Hirano, C.; Imae, T.; Tamura, M.; Takaguchi, Y. Fabrication and Luminescent Properties of Silver Nanoparticles Passivated by Fullerodendrons. Chem. Lett 2005, 34, 862–863. [Google Scholar]
- Murata, Y.; Ito, M.; Komatsu, K. Synthesis and Properties of Novel Fullerene Derivatives Having Dendrimer Units and the Fullerenyl Anions Generated Therefrom. J. Mater. Chem 2002, 12, 2009–2020. [Google Scholar]
- Kunieda, R.; Fujitsuka, M.; Ito, O.; Ito, M.; Murata, Y.; Komatsu, K. Photochemical and Photophysical Properties of C60 Dendrimers Studied by Laser Flash Photolysis. J. Phys. Chem 2002, 106, 7193–7199. [Google Scholar]
- Donnio, B.; Buathong, S.; Bury, I.; Guillon, D. Liquid Crystalline Dendrimers. Chem. Soc. Rev 2007, 36, 1495–1513. [Google Scholar]
- Campidelli, S.; Severac, M.; Scanu, D.; Deschenaux, R.; Vazquez, E.; Milic, D.; Prato, M.; Carano, M.; Marcaccio, M.; Paolucci, F.; Rahman, G.M.A.; Guldi, D.M. Photophysical, Electrochemical, and Mesomorphic Properties of a Liquid-crystalline [60]Fullerene–peralkylated Ferrocene Dyad. J. Mater. Chem 2008, 18, 1504–1509. [Google Scholar]
- Nierengarten, J.F.; Guttierez-Nava, M.; Zhang, S.; Masson, P.; Oswald, L.; Bourgogne, C.; Rio, Y.; Accorsi, G.; Armaroli, N.; Setayesh, S. Fullerene-containing Macromolecules for Materials Science Applications. Carbon 2004, 42, 1077–1083. [Google Scholar]
- Zhang, S.; Rio, Y.; Cardinali, F.; Bourgogne, C.; Gallani, J.L.; Nierengarten, J.F. Amphiphilic Diblock Dendrimers with a Fullerene Core. J. Org. Chem 2003, 68, 9787–9797. [Google Scholar]
- Choi, M.S.; Aida, T.; Luo, H.; Araki, Y.; Ito, O. Fullerene-Terminated Dendritic Multiporphyrin Arrays: Dendrimer Effects on Photoinduced Charge Separation. Angew. Chem. Int. Ed 2003, 42, 4060–4063. [Google Scholar]
- Negishi, N.; Ie, Y.; Tada, H.; Kaneda, T.; Aso, Y. Ambipolar Characteristics of Dendritic Oligothiophene/Fullerene Linkage Molecules. Chem. Lett 2007, 36, 544–545. [Google Scholar]
- Scanu, D.; Yevlampieva, N.P.; Deschenaux, R. Polar and Electrooptical Properties of [60] Fullerene-Containing Poly (benzyl ether) Dendrimers in Solution. Macromolecules 2007, 40, 1133–1139. [Google Scholar]
- Yamaguchi, T.; Ishii, N.; Tashiro, K.; Aida, T. Supramolecular Peapods Composed of a Metalloporphyrin Nanotube and Fullerenes. J. Am. Chem. Soc 2003, 125, 13934–13935. [Google Scholar]
- Nishioka, T.; Tashiro, K; Aida, T.; Zheng, J.Y.; Kinbara, K.; Saigo, K.; Sakamoto, S.; Yamaguchi, K. Molecular Design of a Novel Dendrimer Porphyrin for Supramolecular Fullerene/Dendrimer Hybridization. Macromolecules 2000, 33, 9182–9184. [Google Scholar]
- Iliopoulos, K.; Couris, S.; Hartnagel, U.; Hirsch, A. Nonlinear Optical Response of Water Soluble C70 Dendrimers. Chem. Phys. Lett 2007, 448, 243–247. [Google Scholar]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev 1999, 99, 1665–1688. [Google Scholar]
- Takaguchi, Y.; Suzuki, S.; Ohta, K.; Motoyoshiya, J.; Aoyama, H. Synthesis and Characterization of a Poly (benzyl ether) Dendron Sulfenyl Iodide. Phosphor. Sulfur Silicon 2001, 176, 61–67. [Google Scholar]
- Hecht, S.; Fréchet, J.M.J. Dendritic Encapsulation of Function: Applying Nature’s Site Isolation Principle from Biomimetics to Materials Science. Angew. Chem. Int. Ed 2001, 40, 74–91. [Google Scholar]
- Stefarn, B.; Marcel, K.; Volker, E.; Martin, B.; Klaus, M. Pyrene as Chromophore and Electrophore: Encapsulation in a Rigid Polyphenylene Shell. Chem. Eur. J 2006, 12, 6117–6128. [Google Scholar]
- Amore, A.; Heerbeek, R.V.; Zeep, N.; Esch, J.V.; Reek, J.N.H.; Hiemsta, H.; Maarseveen, J.H.V. Carbosilane Dendrimeric Carbodiimides: Site Isolation as a Lactamization Tool. J. Org. Chem 2006, 71, 1851–1860. [Google Scholar]
- Kumagai, N.; Matsunaga, S.; Shibasaki, M. An Efficient Synthesis of Bicyclic Amidines by Intramolecular Cyclization. Angew. Chem. Int. Ed 2004, 43, 478–482. [Google Scholar]
- Fukuzumi, S.; Suenobu, T.; Hirasaka, T.; Sakurada, N.; Arakawa, R.; Fujitsuka, M.; Ito, O. Enhanced Reactivity of C70 in the Photochemical Reactions with NADH and NAD Dimer Analogues as Compared to C60 via Photoinduced Electron Transfer. J. Phys. Chem. A 1999, 103, 5935–5941. [Google Scholar]
- Roduner, E.; Reid, I.D. Structure and Charge Distribution of Multiply Charged C70. Chem. Phys. Lett 1994, 223, 149–154. [Google Scholar]



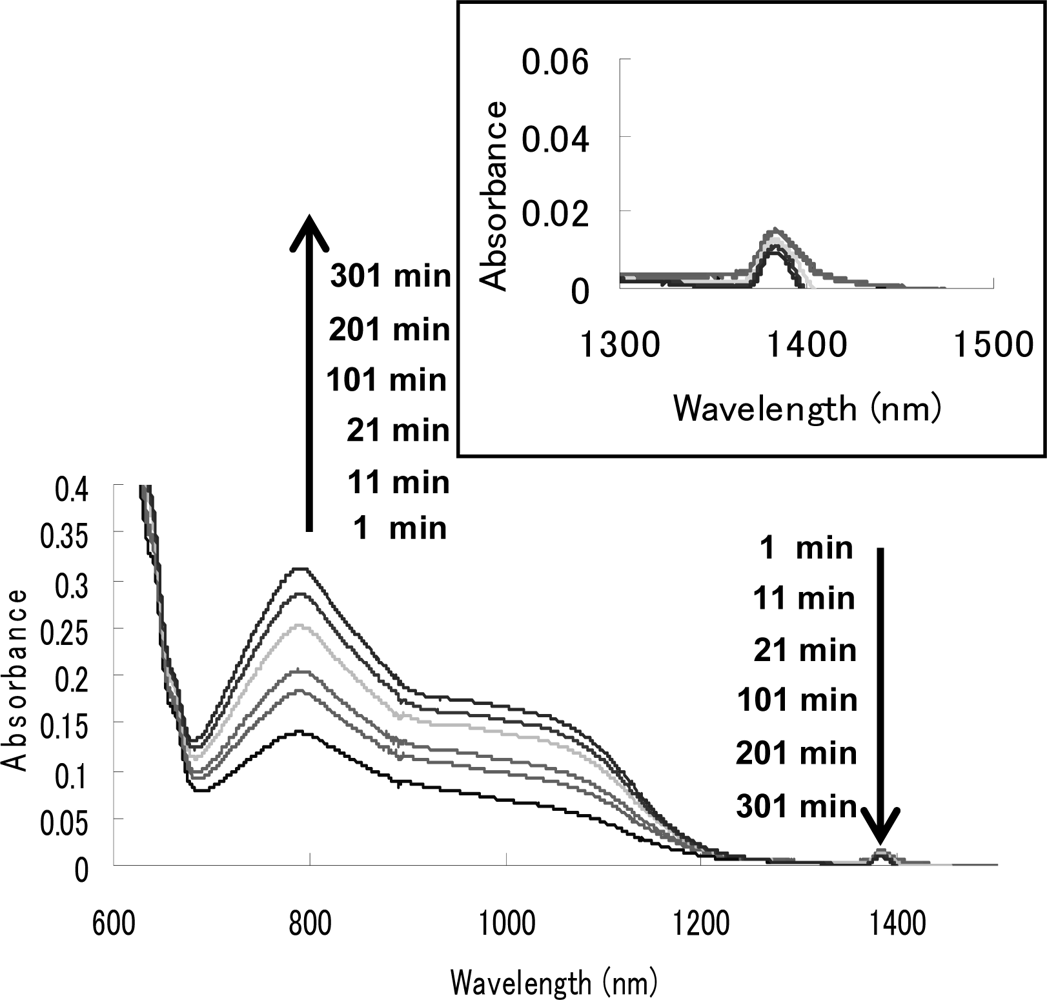

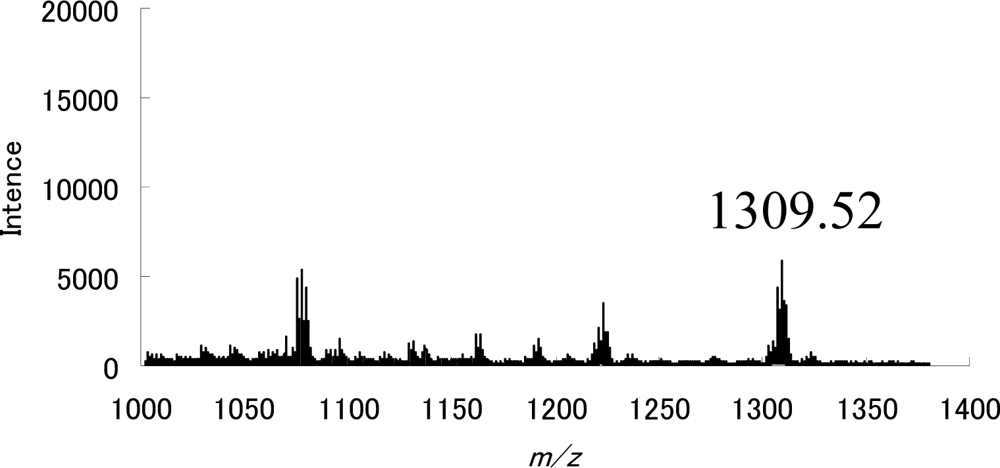



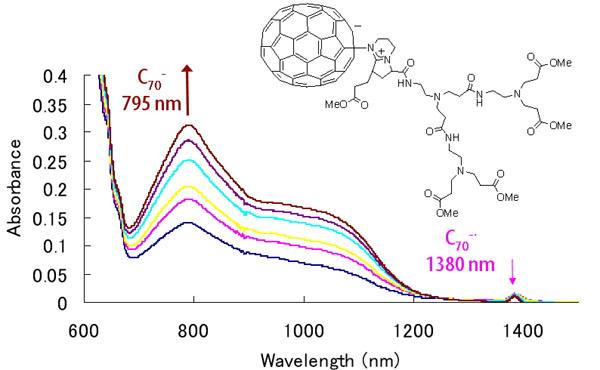
| Compound | λmax [nm] | half-life [min] a |
|---|---|---|
| 7 | 930 | 377 |
| 9a | 930 | 397 |
| 9b | 930 | 463 |
| 10 | 795 | 1445 |
| 12a | 795 | 4800 |
| 12b | 795 | 7345 |
©2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Takaguchi, Y.; Hosokawa, M.; Mayahara, M.; Tajima, T.; Sasamori, T.; Tokitoh, N. Formation of Zwitterionic Fullerodendron Using a New DBN-Focal Dendron. Sensors 2010, 10, 613-624. https://doi.org/10.3390/s100100613
Takaguchi Y, Hosokawa M, Mayahara M, Tajima T, Sasamori T, Tokitoh N. Formation of Zwitterionic Fullerodendron Using a New DBN-Focal Dendron. Sensors. 2010; 10(1):613-624. https://doi.org/10.3390/s100100613
Chicago/Turabian StyleTakaguchi, Yutaka, Maki Hosokawa, Masatoshi Mayahara, Tomoyuki Tajima, Takahiro Sasamori, and Norihiro Tokitoh. 2010. "Formation of Zwitterionic Fullerodendron Using a New DBN-Focal Dendron" Sensors 10, no. 1: 613-624. https://doi.org/10.3390/s100100613