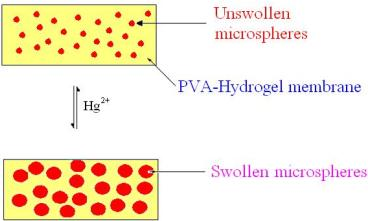
Optical Sensing Properties of Dithiocarbamate-Functionalized Microspheres, Using a Polyvinylpyridine-Polyvinylbenzyl Chloride Copolymer
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Instruments
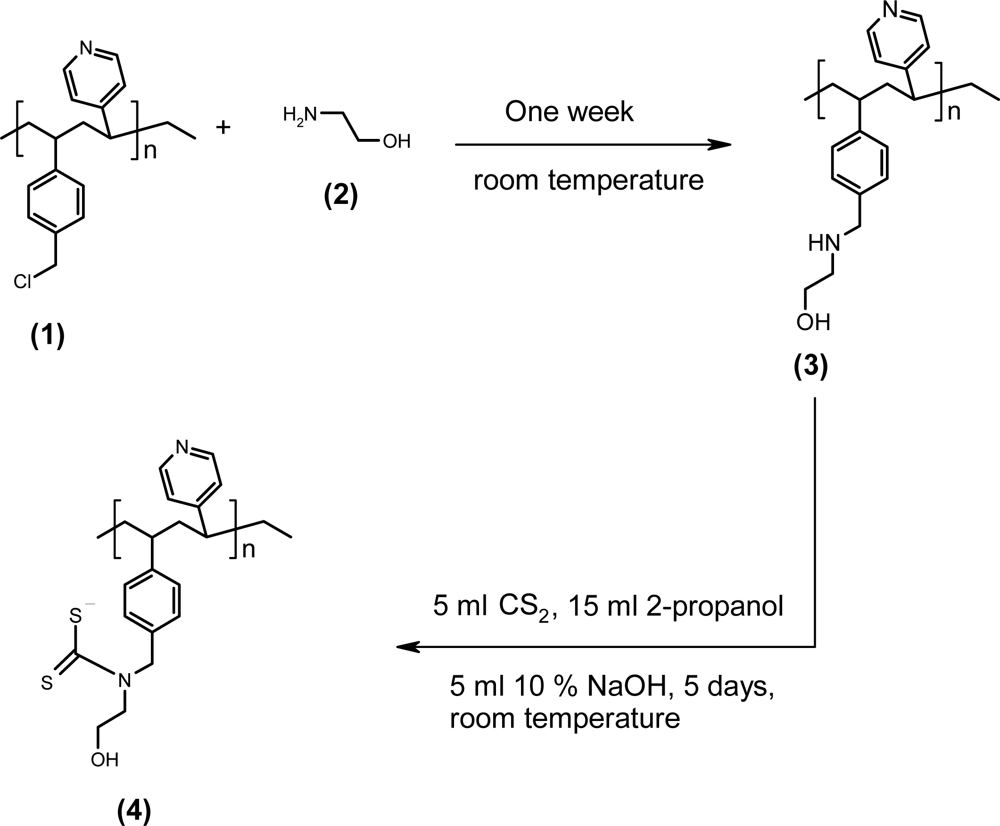
2.3. Synthesis of the dithiocarbamate polymer
2.4. Polymer capacity
2.5. Optical measurements
3. Results and Discussion
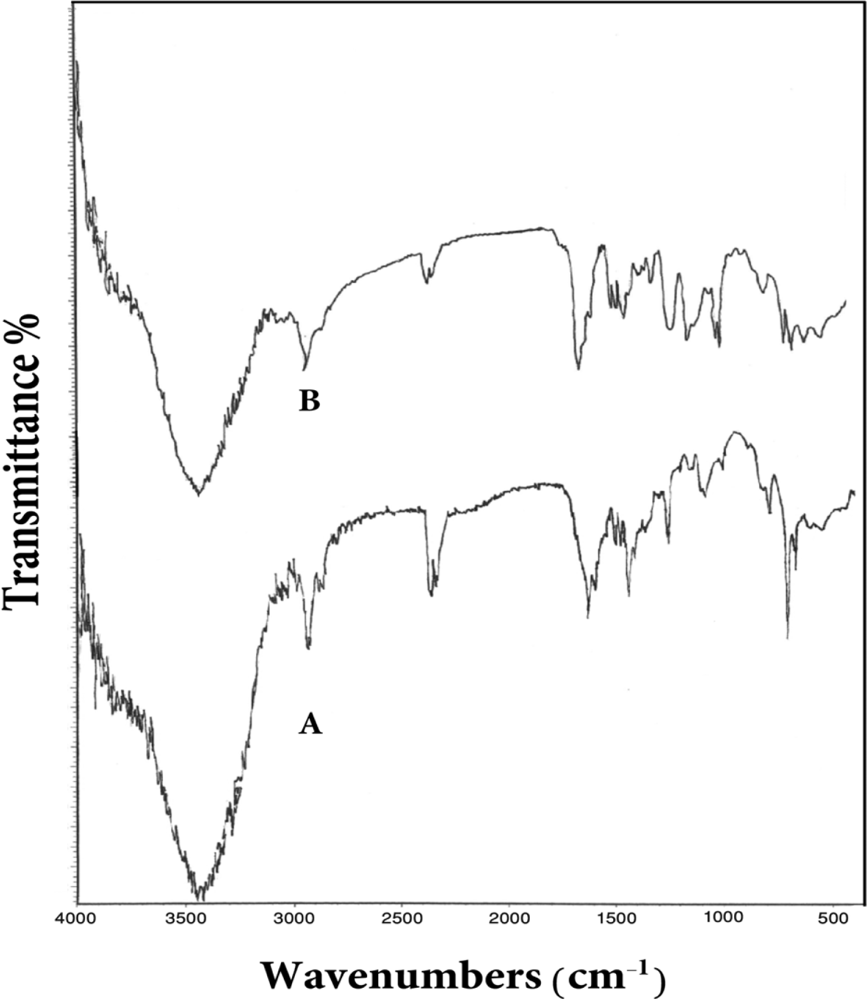
3.1. Characterization of the dithiocarbamate polymer
3.2. Sensor evaluation
4. Conclusions
Acknowledgments
References
- McCurley, MF; Seitz, WR. Fiber-optic sensor for salt concentration based on polymer swelling coupled to optical displacement. Anal. Chim. Acta 1991, 249, 373–380. [Google Scholar]
- Odeh, IMA; Siam, S; Katib, M; Shakhsher, Z. An optical chemical sensor based on polymer swelling and shrinking using dithiocarbamate-polymer microspheres. Jordan J. Chem 2009, 4, 55–64. [Google Scholar]
- Rooney, MT; Seitz, WR. An optically sensitive membrane for pH based on swellable polymer microspheres in a hydrogel. Anal. Comm 1999, 36, 267–270. [Google Scholar]
- Seitz, WR; Rooney, MT; Miele, EW; Wang, H; Kaval, N; Zhang, L; Doherty, S; Milde, S; Lenda, J. Derivatized swellable polymer microspheres for chemical transduction. Anal. Chim. Acta 1999, 400, 55–64. [Google Scholar]
- Shakhsher, Z; Seitz, WR; Legg, KD. Single fiber-optic pH sensor based on changes in reflection accompanying polymer swelling. Anal. Chem 1994, 66, 1731–1735. [Google Scholar]
- Shakhsher, Z; Odeh, I; Jaber, S; Seitz, WR. An optical chemical sensor based on swellable dicarboxylate functionalized polymer microspheres for pH copper and calcium determination. Microchim. Acta 2004, 144, 147–153. [Google Scholar]
- Zhang, Z; Shakhsher, Z; Seitz, WR. Aminated polystyrene membranes for a fiber optic pH sensor based on reflectance changes accompanying polymer swelling. Mikrochim. Acta 1995, 121, 41–50. [Google Scholar]
- Holtz, JH; Asher, SA. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar]
- Liu, K; Ji, HF. Detection of Pb2+ using a hydrogel swelling microcantilever sensor. Anal. Sciences 2004, 20, 9–11. [Google Scholar]
- Oktar, O; Caglar, P; Seitz, WR. Chemical modulation of thermosensitive poly (N-isopropylacrylamide) microspheres swelling: A new strategy for chemical sensing. Sens. Actuat. B 2005, 104, 179–185. [Google Scholar]
- Leyden, DE; Luttrell, GH. Preconcentration of trace metals using chelating groups immobilized via silylation. Anal. Chem 1975, 47, 1612–1617. [Google Scholar]

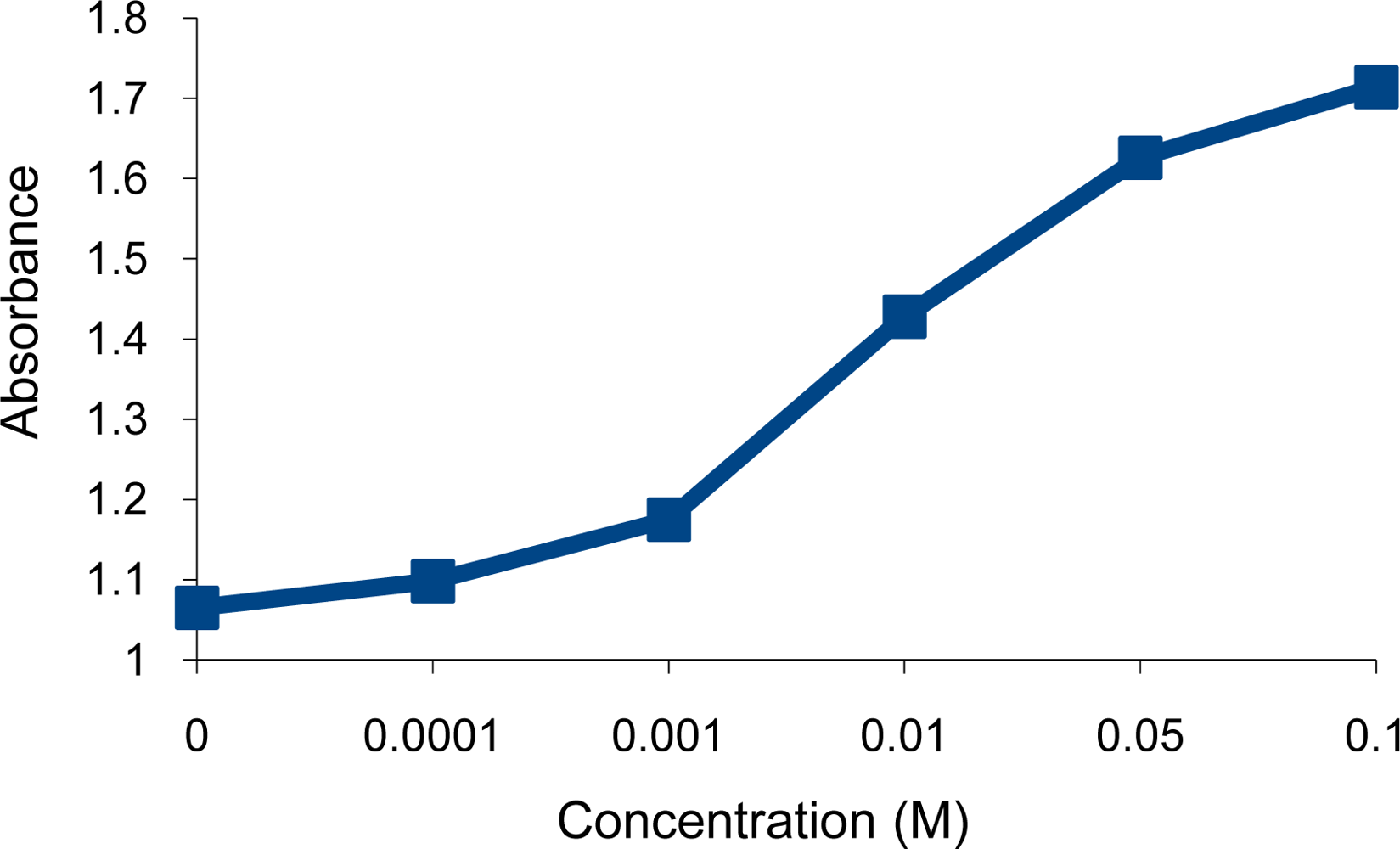
 ), Ni2+ (
), Ni2+ (
 ) and Cr3+ (
) and Cr3+ (
 ) ions.
) ions.
 ), Ni2+ (
), Ni2+ (
 ) and Cr3+ (
) and Cr3+ (
 ) ions.
) ions.
 ), Cd2+ (
), Cd2+ (
 ) and Pb2+ (
) and Pb2+ (
 ) ions.
) ions.
 ), Cd2+ (
), Cd2+ (
 ) and Pb2+ (
) and Pb2+ (
 ) ions.
) ions.


 ), two (
), two (
 ) and three (
) and three (
 ) weeks.
) weeks.
 ), two (
), two (
 ) and three (
) and three (
 ) weeks.
) weeks.
 ), K+(
), K+(
 ) and alkaline earth; Mg2+(
) and alkaline earth; Mg2+(
 ), Ca2+(
), Ca2+(
 ) metal ions.
) metal ions.
 ), K+(
), K+(
 ) and alkaline earth; Mg2+(
) and alkaline earth; Mg2+(
 ), Ca2+(
), Ca2+(
 ) metal ions.
) metal ions.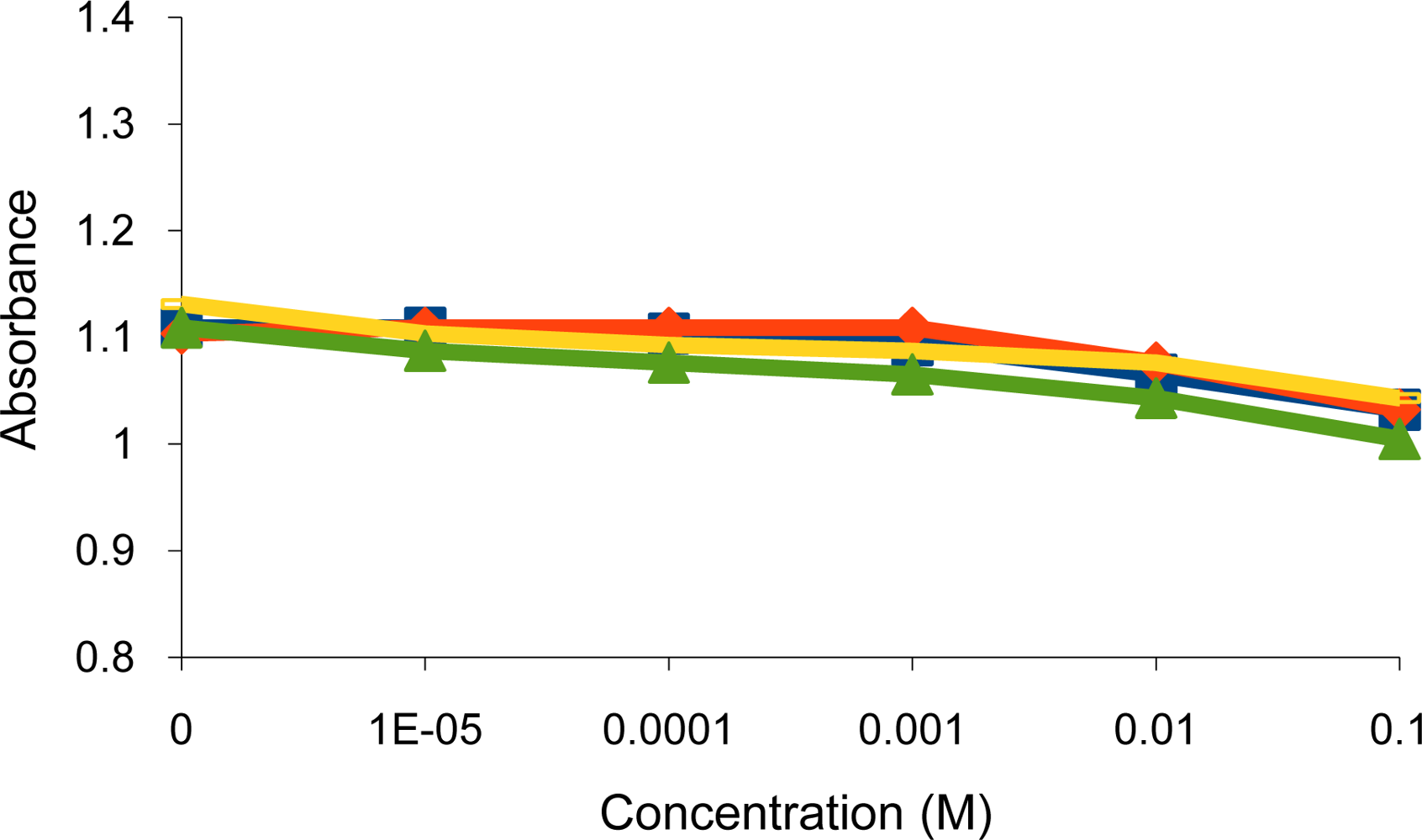
 ) and distilled water (
) and distilled water (
 ).
).
 ) and distilled water (
) and distilled water (
 ).
).
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shakhsher, Z.M.; Odeh, I.M.A.; Rajabi, I.M.S.; Khatib, M.K. Optical Sensing Properties of Dithiocarbamate-Functionalized Microspheres, Using a Polyvinylpyridine-Polyvinylbenzyl Chloride Copolymer. Sensors 2010, 10, 8953-8962. https://doi.org/10.3390/s101008953
Shakhsher ZM, Odeh IMA, Rajabi IMS, Khatib MK. Optical Sensing Properties of Dithiocarbamate-Functionalized Microspheres, Using a Polyvinylpyridine-Polyvinylbenzyl Chloride Copolymer. Sensors. 2010; 10(10):8953-8962. https://doi.org/10.3390/s101008953
Chicago/Turabian StyleShakhsher, Ziad M., Imad M.A. Odeh, Inas M.S. Rajabi, and Mahmoud K. Khatib. 2010. "Optical Sensing Properties of Dithiocarbamate-Functionalized Microspheres, Using a Polyvinylpyridine-Polyvinylbenzyl Chloride Copolymer" Sensors 10, no. 10: 8953-8962. https://doi.org/10.3390/s101008953