A Sulfur Hexafluoride Sensor Using Quantum Cascade and CO2 Laser-Based Photoacoustic Spectroscopy
Abstract
:1. Introduction
2. Methodology
2.1. CO2 Laser Photoacoustic Spectroscopy
2.2. QC Laser Photoacoustic Spectroscopy
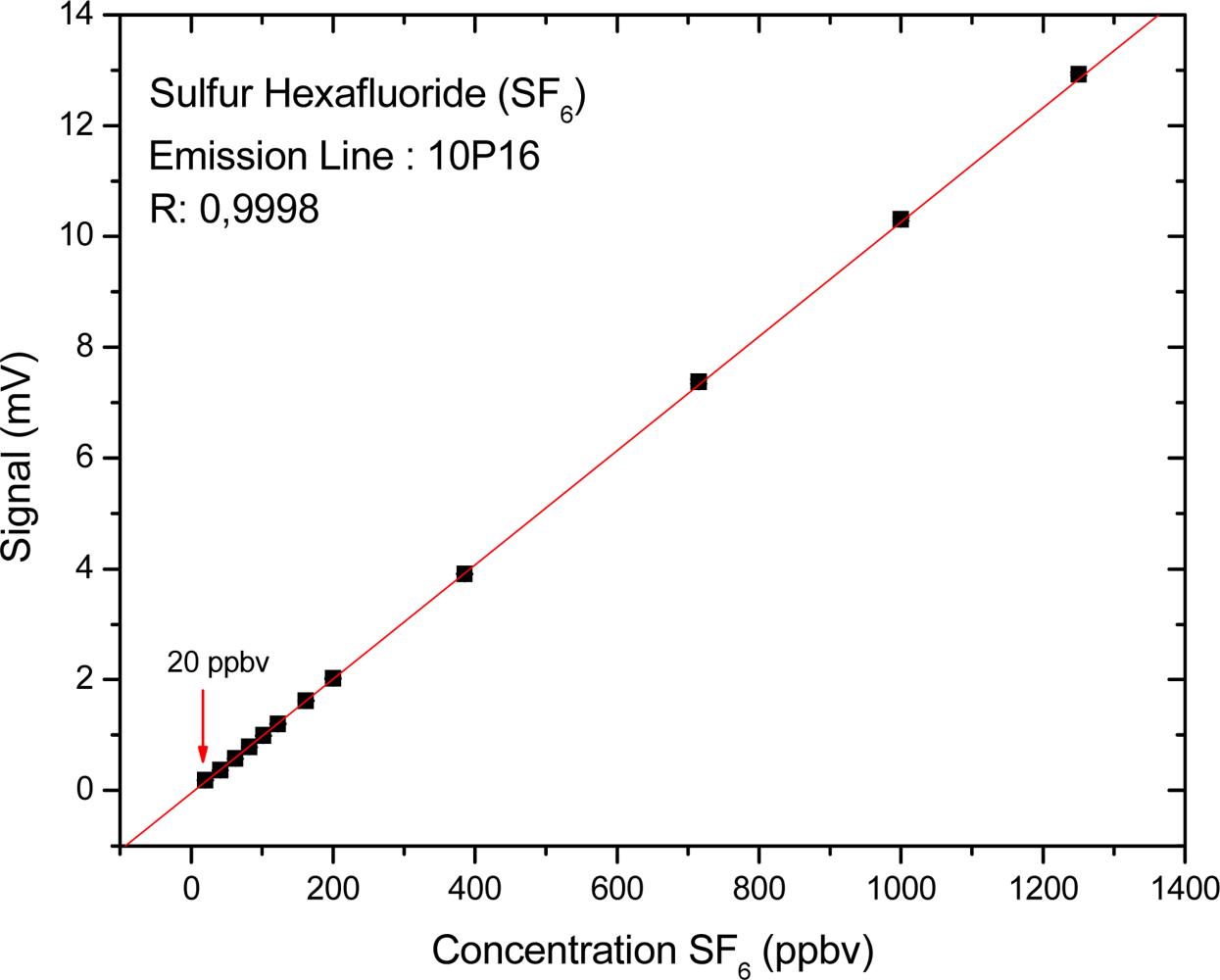
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- IPCC. Report of Working Group I. Climate Change 2007; The Physical Science Basis: Paris, France, February 2007. [Google Scholar]
- Hansen, J; Sato, M; Kharecha, P; Beerling, D; Berner, R; Masson-Delmotte, V; Pagai, M; Raymo, M; Royer, DL; Zachos, JC. Target Atmosphere CO2: Where Should Humanity Aim. Open Atmos. Sci. J 2008, 2, 217–231. [Google Scholar]
- Gillett, NP; Stone, DA; Stott, PA; Nozawa, T; Karpechko, AY; Hegerl, GC; Wehner, MF; Jones, PD. Attribution of Polar Warming to Human Influence. Nat. Geosci 2008, 1, 750–754. [Google Scholar]
- Sander, BD. Forced and Ungforced Ocean Temperature Changes in Atlantic and Pacific Tropical Cyclogenesis Regions. PNAS 2006, 103, 13905–13910. [Google Scholar]
- Emanuel, K. Increasing Destructiveness of Tropical Cyclones over the Past 30 Years. Nature 2005, 436, 686–688. [Google Scholar]
- Kurz, WA. Mountain Pine Beetle and Forest Carbon Feedback to Climate Change. Nature 2008, 452, 987–990. [Google Scholar]
- Greene, CH; Monger, BC; McGarry, LP. Some Like It Cold, Northern Shrimp Stocks Thrive When Climatic Conditions Lead to Cold Bottom Waters. Science 2009, 324, 733–734. [Google Scholar]
- Meinshausen, M. Greenhouse Gas Emission Targets for Limiting Global Warming to 2 °C. Nature 2009, 458, 1158–1163. [Google Scholar]
- Allen, MR; Frame, DJ; Huntingford, C; Jones, CD; Lowe, JA; Meinshausen, M; Meinshausen, N. Warning Caused by Cumulative Carbon Emissions towards the Trillionth Tonne. Nature 2009, 458, 1163–1166. [Google Scholar]
- World Meteorological Organization. Available online: http://www.wmo.int/pages/index_en.html (accessed on November 2009).
- Inventory of US Greenhouse Gas Emissions and Sinks: 1990/2008; U.S. Environmental Protection Agency: Washington, WA, USA, April 2010.
- Sigrist, MW. Trace Gas Monitoring with Infrared Laser-Based Detection Schemes. Appl. Phys. B 2008, 90, 289–300. [Google Scholar]
- Rocha, MV; Sthel, M; Silva, MG; Schramm, DU; Vargas, H. Greenhouse Gases Detection with Quantum Cascade Laser Photoacustic Spectroscopy. Proceedings of Air & Waste Manager’s 103rd Conference, Calgary, Canada, June 2010. in press.
- Cristescu, SM; Persijn, ST; Hekkert, SL; Harren, FJM. Laser-Based Systems for Trace Gas Detection in Life Sciences. Appl. Phys. B 2008, 92, 343–349. [Google Scholar]
- Nagele, M; Sigrist, MW. Mobile Laser Spectrometer with Novel Resonant Multipass Photoacoustic Cell for Trace-Gas Sensing. Appl. Phys. B 2000, 70, 895–901. [Google Scholar]
- Miklos, A; Hess, P; Bozóki, Z. Application of Acoustic Resonators in Photoacoustic Trace Gas Analysis and Metrology. Rev. Sci. Inst 2001, 4, 1937–1955. [Google Scholar]
- Sthel, MS; Schramm, DU; Faria, RT, Jr; Castro, MPP; Carneiro, LO; Ribeiro, WS; Vargas, H. Photoacoustic Spectroscopy-Based Analysis of Gas Samples in A Bus Station. J. Phys. IV France 2005, 125, 881–883. [Google Scholar]
- Lima, JAP. Photoacoustic Detection of NO2 and N2O Using Quantum Cascade Lasers. Appl. Phys. B 2006, 85, 279–284. [Google Scholar]
- Da Silva, M; Miklos, A; Hess, P. Photoacustic Measurement of N2O Concentration in Ambient Air with a Pulsed Optical Parametric Oscillator. Appl. Phys 2006, 82, 329–336. [Google Scholar]
- Schramm, DUS; Sthel, MS; Silva, MG; Carneiro, LO; Junior, AJS; Vargas, H. Application of Laser Photoacoustic Spectroscopy for The Analysis of Gas Samples Emitted by Diesel Engines. Inf. Phys. Technol 2003, 44, 263–269. [Google Scholar]
- Gondal, MA; Dastageer, A; Shwehdi, MH. Photoacustic Spectrometry for Trace Gas Analysis and Leak Detection Using Different Cell Geometries. Talanta 2004, 62, 131–141. [Google Scholar]
- Gondal, MA; Baig, MA; Shwehdi, MH. Laser Sensor for Detection of SF6 Leaks in High Insulated Switch Gear Systems. IEEE Trans. Dieletrics Eletrical Insulations 2002, 9, 421–427. [Google Scholar]
- Gondal, MA; Yamani, ZH. High Sensitive Electronically Modulated Photoacoustic Spectrometer for Ozone Detection. Appl. Opt 2008, 46, 7083–7090. [Google Scholar]
- Kosterev, AA; Curl, RF; Tittel, FK; Rochat, M; Beck, M; Hofstetter, D; Faist, J. Chemical Sensing with Pulsed QC-DFB Lasers Operating at 15.6 μm. Appl. Phys. B 2002, 75, 351–357. [Google Scholar]
- Meyer, T; Braun, M; Lambrecht, A. Fast Gas Spectroscopy Using Pulsed Quantum Cascade Lasers. J. Appl. Phys 2003, 93, 3158–3160. [Google Scholar]
- Silva, MG; Vargas, H; Miklós, A; Hess, P. Photoacoustic Detection of Ozone Using Quantum Cascade Laser. Appl. Phys. B 2004, 78, 1513–1516. [Google Scholar]
- Curl, RF; Capasso, F; Gmachl, C; Kosterev, AA; McManus, B; Lewicki, R; Pusharsky, M; Wysocki, G; Tittel, FK. Quantum Cascade Lasers in Chemical Physics. Chem. Phys. Lett 2010, 487, 1–18. [Google Scholar]
- Baptista, FM; Da Silva, MG; Sthel, MS; Schramm, DU; Vargas, H; Miklos, A; Hess, P. Ammonia Detection Using Quantum-Cascade Laser Photoacoustic Spectroscopy. Appl. Opt 2006, 45, 4966–4971. [Google Scholar]
- Zeninary, V; Grossel, A; Joly, L; Decarpenterie, T; Grouiez, B; Bonno, B; Parvitte, B. Photoacoustic Spectroscopy for Trace Gas Detection with Cryogenic for Trace Gas Detection with Cryogenic and Room-Temperature Continuous-Wave Quantum Cascade Lasers. Cent. Eur. J. Phys 2010, 8, 194–201. [Google Scholar]
- Killinger, DA; Mooradian, A. Optics and Laser Remote Sensing Springer Series in Optical Science; Springer-Verlag: Berlin, Germany, 1988. [Google Scholar]
- Measures, RM. Laser Remote Chemical Analysis; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Thony, M; Sigrist, W. New Developments in CO2-Laser Photoacoustic Monitoring of Trace Gases. Infrared Phys. Technol 1995, 36, 585–615. [Google Scholar]
- Bijnen, FG; Reuss, CJ; Harren, F. Geometrical Optimization of a Longitudinal Resonant Photoacoustic Cell for Sensitive and Fast Trace Gas Detection. J. Rev. Sci. Inst 1996, 67, 2914–2923. [Google Scholar]
- Pereira, T; Almeida, PSG; Da Silva, MG; Vargas, H. Gas Diffusion in ‘Golden’ Papaya Fruit at Different Maturity Stages. Postharvest Biol. Thechnol 2009, 54, 123–130. [Google Scholar]
- National Oceanic and Atmospheric Administration Earth System Research Laboratory—Global Warming Division. Available online: http://www.esrl.noaa.gov/gmd/hats/insitu/cats/conc/mlosf6.




© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rocha, M.; Sthel, M.; Lima, G.; Silva, M.d.; Schramm, D.; Miklós, A.; Vargas, H. A Sulfur Hexafluoride Sensor Using Quantum Cascade and CO2 Laser-Based Photoacoustic Spectroscopy. Sensors 2010, 10, 9359-9368. https://doi.org/10.3390/s101009359
Rocha M, Sthel M, Lima G, Silva Md, Schramm D, Miklós A, Vargas H. A Sulfur Hexafluoride Sensor Using Quantum Cascade and CO2 Laser-Based Photoacoustic Spectroscopy. Sensors. 2010; 10(10):9359-9368. https://doi.org/10.3390/s101009359
Chicago/Turabian StyleRocha, Mila, Marcelo Sthel, Guilherme Lima, Marcelo da Silva, Delson Schramm, András Miklós, and Helion Vargas. 2010. "A Sulfur Hexafluoride Sensor Using Quantum Cascade and CO2 Laser-Based Photoacoustic Spectroscopy" Sensors 10, no. 10: 9359-9368. https://doi.org/10.3390/s101009359



