A Ferrocene-Quinoxaline Derivative as a Highly Selective Probe for Colorimetric and Redox Sensing of Toxic Mercury(II) Cations
Abstract
: A new chemosensor molecule 3 based on a ferrocene-quinoxaline dyad recognizes mercury (II) cations in acetonitrile solution. Upon recognition, an anodic shift of the ferrocene/ferrocenium oxidation peaks and a progressive red-shift (Δλ = 140 nm) of the low-energy band, are observed in its absorption spectrum. This change in the absorption spectrum is accompanied by a colour change from orange to deep green, which can be used for a “naked-eye” detection of this metal cation.1. Introduction
The design and synthesis of chemosensors for environmentally and biologically relevant species have been actively investigated in recent years [1–3]. In this regard, chemosensors that can highly sensitively and selectively monitor heavy metal ions are especially important. Among heavy and transition metals, mercury, widely distributed in air, water and soil, is considered to be one of the highly toxic because both elemental and ionic mercury can be converted by bacteria in the environment to methyl mercury, which subsequently bioaccumulates through the food chain [4–11]. Mercury-induced toxicity can cause a number of severe health problems because it can damage the digestive organs, kidneys, central nervous system and endocrine system [12–17]. Given its high toxicity and the increasing threat of global mercury release into the environment, considerable efforts are continuously made to develop highly selective and sensitive chemosensors for Hg(II). In this context, development of new and practical chemosensors which offer a promising approach for mercury ion detection is still a great challenge for the scientific community [18–23], triggering a large number of related investigations that have been recently reviewed [24–26].
Ferrocene is one of the favourite “building blocks” in the construction of sensing platforms based on redox-active units due to the availability, stability and tailorability of most of its derivatives. The sensing behaviour of these systems is mainly based on the potential shift shown upon their interaction with a variety of guest species. However, binding can also affect the UV-vis properties of the ferrocene unit when it is placed near the binding site. In general, metal complexation induces bathochromic shifts in the lower-energy, spin-allowed ferrocene absorption band, which is between 400 and 500 nm [27–30]
On the other hand, quinoxaline derivatives are the subject of considerable interest from both academic and industrial perspectives because they are significant intermediates for the manufacture of pharmaceuticals and advanced materials [31–34] Moreover, the quinoxaline ring appropriately subtitued or fused to some other azaheterocyclic systems has also been studied as a putative binding subunit for the recognition and sensing of both anionic and cationic especies [35–37]
The work presented here, forms part of our interest in designing chemosensors that are capable of reporting on the recognition of metal cations through a variety of physical responses, by combining various signalling units into an individual molecule. Toward this end, we report here a straightforward synthesis of the new 2,3-diferrocenylquinoxaline ligand which shows a selective, sensitive and reversible response to the Hg(II) ion through two different channels: redox and chromogenic
2. Experimental Section
All reactions were carried out using solvents which were dried by routine procedures. The melting point was determined on a hot-plate melting point apparatus and is uncorrected. 1H- and 13C-NMR spectra were recorded at 400 and 100 MHz, respectively on a Brucker AC 400. The following abbreviations for stating the multiplicity of the signals have been used: s (singlet), bs, d (doublet), t (triplet), st (pseudotriplet), and q (quaternary carbon atom). Chemical shifts refer to signals of tetramethylsilane in the case of 1H- and 13C-NMR spectra. The cyclic electrochemistry measurements were performed on a Bioanalytical Systems CV-50 W Voltammetric Analyzer potentiostat/galvanostat controlled by a personal computer and driven by dedicated software with a conventional three-electrode configuration consisting of platinum working and auxiliary electrodes and an SCE reference electrode. The experiments were carried out with a 10−3 M solution of sample in dry CH3CN containing 0.1 M [(n-Bu)4N]ClO4 as supporting electrolyte (Warning: Potential formation of highly explosive perchlorate salts of organic derivatives). Deoxygenation of the solutions was achieved by bubbling nitrogen for at least 10 min, and the working electrode was cleaned after each run. The cyclic voltammograms were recorded with a scan rate between 0.05 and 0.5 V s−1. Linear sweep voltammetry (LSV), cyclic voltammetry (CV), and Osteryoung square wave voltammetry (OSWV) were recorded before and after the addition of aliquots of 0.1 equiv of 2.5 × 10−2 M solutions of the corresponding cations in H2O. The following settings were used: pulse amplitude, 50 mV; pulse width, 50 ms; scan rate, 100 mV/s; sample width, 17 ms; pulse period, 200 ms. Decamethylferrocene (DMFe) (−0.07 V vs SCE) was used as an internal reference both for potential calibration and for reversibility criteria. UV-vis absorption spectra were regularly recorded after the addition a small aliquot of the corresponding cation (c = 2.5 × 10−3 M) to a solution of the receptor (c = 1 × 10−4 M) using a UV quartz cell.
2.1. Preparation of 2,3-diferrocenylquinoxaline (3)
2,3-Diaminobenzene (1, 77 mg, 0.7 mmol) was added to a solution of diferrocenylethane-1,2-dione (2, 0.3 g, 0.7 mmol) in ethanol (50 mL). The mixture was stirred under reflux overnight during which time an orange solid precipitated, which was isolated by filtration, washed with cold diethyl ether (3 × 10 mL) and finally crystallized in ethanol. Yield 98%. M.p > 300 °C. 1H-NMR (CD3CN): δ 4.09 (s, 10H), 4.32 (st, 4H), 4.64 (st, 4H), 7.67 (dd, 2H, J = 3.4 Hz, J = 6.4 Hz), 8.03 (dd, 2H, J = 3.4 Hz, J = 6.4 Hz); 13C-NMR (CDCl3): δ 68.7 (4xCH), 69.7 (10xCH), 71.4 (4xCH), 85.2 (2xq), 128.5 (2xCH), 128.7 (2xCH), 140.4 (2xq), 152.9 (2xq); FAB MS: m/z (relative intensity): 498 (M+,100); Anal Calc for C28H22Fe2N2: C, 67.57; H, 4.45; N, 5.62. Found: C, 67.80; H, 4.82; N, 5.40.
3. Results and Discussion
3.1. Synthesis
The quinoxaline-based receptor 3 was prepared following the classical method for synthesizing both quinoxaline itself and its derivatives, which involves the condensation of an aromatic 1,2-diamine with a 1,2-dicarbonyl compound in refluxing ethanol or acetic acid (Scheme 1) [38]. Thus, condensation of the readily available diferrocenylethane-1,2-dione (2) [35] with 1,2-diaminobenzene (1) gave an excellent yield (98%) of the corresponding 2,3-diferrocenylquinoxaline (3) which was fully characterized by using standard techniques: 1H-NMR and 13C-NMR spectroscopies, FAB mass spectrometry and elemental analysis.
3.2. Electrochemical and Optical Properties
The redox properties of receptor 3 was investigated by linear sweep voltammetry (LSV), cyclic voltammetry (CV), and Osteryoung square wave voltammetry (OSWV) in a CH3CN solution containing 0.15 M [n-Bu4N]ClO4 (TBAP) as supporting electrolyte. In spite of the symmetry of the receptor 3 it exhibited, in the range 0−0.9 V, two reversible one-electron redox wave at the half-wave potential value of 1E1/2 = 0.47 V and 2E1/2 = 0.58 V (ΔE1/2 = 110mV) versus decamethylferrocene (DMFc), demonstrating the existence of a weak interaction between the two iron centres (Figure 1). The criteria applied for reversibility was a separation of ∼60 mV between cathodic and anodic peaks, a ratio of 1.0 ± 0.1 for the intensities of the cathodic and anodic currents Ic/Ia, and no shift of the half-wave potentials with varying scan rates.
The UV−vis spectra for receptor 3 was recorded as 10−4 M solution in CH3CN and contains three prominent absorption bands with a maximum at 234 nm (ɛ = 26,000 M−1 cm−1), 277 nm (ɛ = 14750 M−1 cm−1) and 314 nm (ɛ = 9420 M−1 cm−1) which can safely be ascribed to a high energy ligand-centered π−π* electronic transition (L−π*) (HE band). In addition to this band, another two weaker absorptions are visible at 409 nm (ɛ = 1,590 M−1 cm−1) and 490 nm (ɛ = 1,860 M−1 cm−1) which are assigned to another localized excitations with a lower energy produced either by two nearly degenerate transitions, an Fe(II) d−d transition or by a metal−ligand charge transfer (MLCT) process (dπ−π*) (LE band) [39] This assignment is in accordance with the latest theoretical treatment (model III) reported by Barlow et al. [40]. Such spectral characteristics confer an orange color to this species.
3.3. Cation Sensing Properties
One of the most interesting attributes of the new diferrocenylquinoxaline reported here is the presence of metal-ion binding sites on the quinoxaline ring close to a ferrocene redox-active moiety. Due to this structural feature metal recognition properties on the receptor 3 were evaluated by electrochemical, optical and 1H-NMR techniques.
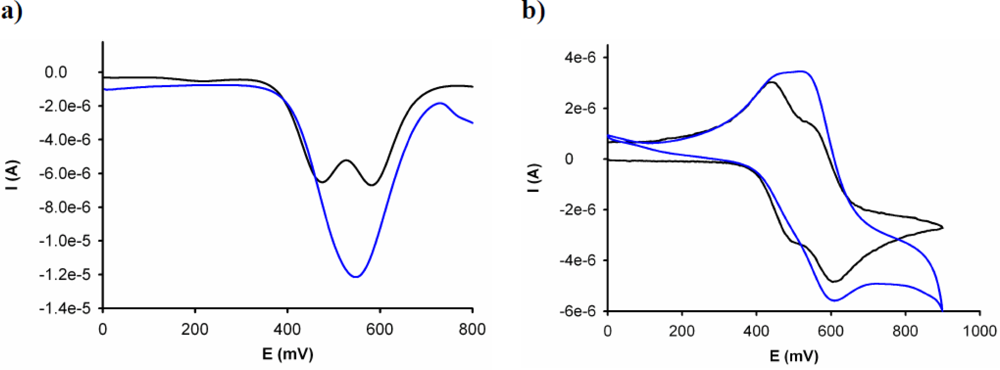
The electrochemical binding interactions of 3 towards cations of biological and environmental relevance, such as Li+, Na+, K+, Ca2+, Mg2+, Cu2+, Zn2+, Cd2+, Hg2+, Ni2+, and Pb2+, added as their perchlorate salts, were investigated in CH3CN (c = 1 × 10−3 M). Titration studies with addition of the above-mentioned set of metal cations (2.5 × 10−2 M in H2O) to an electrochemical solution of receptor 3 containing [n-Bu4N]ClO4 (0.1 M) as supporting electrolyte, demonstrate that while addition of Cu2+ and Hg2+ ions promotes remarkable responses, addition of Li+, Na+, K+, Ca2+, Mg2+, Zn2+, Cd2+, Pb2+ and Ni2+ metal ions had no effect either on LSV or on the CV or OSWV of this receptor, even when present in a large excess. The results obtained on the stepwise addition of substoichiometric amounts of Hg2+ revealed the appearance, in the OSWV, of a new oxidation peak at practically the same potential of the second redox peak in the free receptor (Ep = 0.55 V, ΔEp = 75 mV).This fact suggests that the complex is disrupted after the first monoelectronic oxidation of the complex 3+·Hg2+ and the second oxidation really takes place on the uncomplexed mono-oxidized 3+. The current intensity of this new peak increases until 1 equiv of the Hg2+ cation is added [Figure 2(a)]. Moreover, the CV analysis of the complex 3·Hg2+ shows that one reduction process takes place at the same reduction potential showed by the uncomplexed ligand 3, indicating that the complex starts to be disrupted after its electronic oxidation [Figure 2(b)]. This behaviour means that this receptor is not only able to monitor binding but it is also able to behave as an electrochemically induced switchable chemosensor for Hg2+ through the progressive electrochemical release of these metal cations; as a result of a decrease of the corresponding binding constant upon electrochemical oxidation.
Remarkably, LSV studies carried out upon addition of Cu2+ to the CH3CN solution of this receptor showed a significant shift of the sigmoidal voltammetric wave toward cathodic currents, indicating that Cu2+ cations promote the oxidation of the free receptor. On the other hand, the same experiments carried out upon addition of Hg2+ revealed a shift of the linear sweep voltammogram toward more positive potentials, indicating the complexation process according to the previously observed by OSWV (Figure 3).
Previous studies on ferrocene-based ligands have shown that their characteristic low energy (LE) bands in the absorption spectra are perturbed upon complexation [41–44]. Therefore, the metal recognition properties of the ligand 3 toward metal ions were also evaluated by UV−vis spectroscopy. Titration experiments for CH3CN solutions of this ligand (c = 1 × 10−4 M), and the corresponding cations were performed and analyzed quantitatively. [45] It is worth mentioning that no changes were observed in the UV−vis spectra upon addition of Li+, Na+, K+, Ca2+, Mg2+, Zn2+, Cd2+, and Ni2+ and Pb2+ metal ions, even in a large excess; however, significant modifications were observed upon addition of Hg2+.
Thus, the addition of increasing amounts of Hg2+ ions in water to a solution of 3 caused a decrease in the intensity of the LE band, at λ = 490 nm, along with the progressive appearance of a new band located at λ = 630 nm (ɛ = 790 M−1 cm−1) as well as a increase of the initial HE band intensity. Two well-defined isosbestic points at 439 and 531 indicate that a neat interconversion between the uncomplexed and complexed species occurs [Figure 4(a)]. The new LE band is red-shifted by 140 nm and is responsible for the change of colour, from orange to deep green, which can be used for a “naked-eye” detection of this metal ion [Figure 4(b)]. Binding assays using the method of continuous variations (Job’s plot) suggests a 1:1 binding model (metal/ligand) with a log Ka = 3.4 ± 0.17 [Figure 4(c)]. Moreover, the calculated detection limit [46] was 1.3 × 10−5 M. Additionally the peak corresponding to the complex [3·Hg]2+ was observed by ES-MS at m/z 700.02. The relative abundance of the isotopic clusters was in good agreement with the simulated spectrum of the 1:1 complex.
In order to get additional information about the coordination between the receptor 3 and Hg2+ cations, a 1H-NMR titration experiment was performed where aliquots of metal cation in D2O were added to a solution of the receptor in CD3CN. The free receptor 3 exhibits two sets of signals: one of them corresponding to the ferrocene moiety and another one to the quinoxaline ring. The ferrone moieties show a signal at δ = 4.10 (s), corresponding to the protons present in the unsubstituted ciclopentadienyl (Cp) unit and two psudotriplets at 4.32 and 4.64 ppm assigned to the Hβ, and Hα within the monosubustituted Cp ring. On the other hand, the quinoxaline ring displays two double doublets at δ = 7.67 (H-6) and 8.03 (H-5) ppm. An inspection of the 1H-NMR titration data showed a strong chemical shift for the signals associated with the ferrocene units due to their proximity to the binding sites. The protons within the unsubstituted Cp were shifted Δδ = +0.26 ppm and the Hα and Hβ protons Δδ = 0.59 and 0.52 ppm respectively. On the other hand a weaker shift (Δδ = 0.1 ppm) in the H-5 and H-6 protons of the quinoxaline ring were also observed (Figure 5).
4. Conclusions
We have successfully developed a new and easy-to-make quinoxaline-based molecular sensor 3 which shows selective response to Hg2+ ions through a dual channel: Electrochemical and chromogenic. The reported quinoxaline-ferrocene sensor permits not only the naked-eye detection of this metal cation but also to monitor the recognition process through electrochemical measurements. Additionally, this receptor is also able to behave as an electrochemically induced switchable chemosensor for Hg2+. A combination of the UV-vis titration data and mass spectrometry has been successfully used to establish the 1:1 stoichiometry of the complex formed.
Acknowledgments
We gratefully acknowledge the financial support from MICINN-Spain, Project CTQ2008-01402 and Fundación Séneca (Agencia de Ciencia y Tecnología de la Región de Murcia) project 04509/GERM/06 (Programa de Ayudas a Grupos de Excelencia de la Región de Murcia, Plan Regional de Ciencia y Tecnología. F.Z. and A.C also thank for a postdoctoral contract from the Ministerio de Educación de España (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+I 2008-2011) and a Marie Curie Postdoctoral Fellowship of the European Union, respectively
References and Notes
- de Silva, AP; Gunaratne, HQN; Gunnlaugsson, T; Huxley, AJM; McCoy, CP; Rademacher, JT; Rice, TE. Signaling recognition events with fluorescent sensors and switches. Chem Rev 1997, 97, 1515–1566. [Google Scholar]
- Valeur, B; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 2000, 205, 3–40. [Google Scholar]
- Amendola, V; Fabbrizzi, L; Foti, F; Licchelli, M; Mangano, C; Pallavicini, P; Poggi, A; Sacchi, D; Taglietti, A. Light-emitting molecular devices based on transition metals. Coord Chem Rev 2006, 250, 273–299. [Google Scholar]
- Basu, N; Scheuhammer, A; Grochowina, N; Klenavic, K; Evans, D; O’Brien, M; Chan, H. Effects of mercury on neurochemical receptors in Wild River Otters (Lontra canadensis). Environ Sci Technol 2005, 39, 3585–3591. [Google Scholar]
- Zhang, Z; Wu, D; Guo, X; Qian, X; Lu, Z; Xu, Q; Yang, Y; Duan, L; He, Y; Feng, Z. Visible study of mercuric ion and its conjugate in living cells of mammals and plants. Chem Res Toxicol 2005, 18, 1814–1820. [Google Scholar]
- US EPA. Regulatory Impact Analysis of the Clean Air Mercury Rule, EPA-452/R-05-003. Available online: http://www.epa.gov/ttnecas1/regdata/RIAs/mercury_ria_final.pdf/ (accessed on 20 November 2010).
- Wang, Q; Kim, D; Dionysiou, DD; Sorial, GA; Timberlake, D. Sources and remediation for mercury contamination in aquatic systems—Aliterature review. Environ Pollut 2004, 131, 323–336. [Google Scholar]
- Tchounwou, PB; Ayensu, WK; Ninashvili, N; Sutton, D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 2003, 18, 149–175. [Google Scholar]
- Onyido, I; Norris, AR; Buncel, E. Biomolecule-mercury interactions: Modalities of DNA base-mercury binding mechanisms. Remediation strategies. Chem Rev 2004, 104, 5911–5929. [Google Scholar]
- Feng, XB; Li, P; Qiu, GL; Wang, S; Li, GH; Shang, LH; Meng, B; Jiang, HM; Bai, WY; Li, ZG; Fu, XW. Human exposure to methylmercury through rice intake in mercury Mining areas, Guizhou province, China. Environ Sci Technol 2008, 42, 326–332. [Google Scholar]
- Krupp, EM; Mestrot, A; Wielgus, J; Meharg, AA; Feldmann, J. The molecular form of mercury in biota: identification of novel mercury peptide complexes in plants. Chem Commun 2009, 28, 4257–4259. [Google Scholar]
- Shanker, G; Mutkus, LA; Walker, SJ; Aschner, M. Methylmercury enhances arachidonic acid release and cytosolic phospholipase A2 expression in primary cultures of neonatal astrocytes. Mol Brain Res 2002, 106, 1–11. [Google Scholar]
- Clarkson, TW; Magos, L; Myers, GJ. The toxicology of mercury—Current exposures and clinical manifestations. N Engl J Med 2003, 349, 1731–1737. [Google Scholar]
- Silbergeld, EK; Silva, IA; Nyland, JF. Mercury and autoimmunity: Implications for occupational and environmental health. Toxicol Appl Pharmacol 2005, 207, 282–292. [Google Scholar]
- Clarkson, TW; Magos, L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 2006, 36, 609–662. [Google Scholar]
- Ye, B-C; Yin, B-C. Highly sensitive detection of mercury (II) Ions by fluorescence polarization enhanced by gold nanoparticles. Angew Chem Int Ed 2008, 47, 8386–8389. [Google Scholar]
- Crespo-López, ME; Macêdo, GL; Pereira, SID; Arrifano, GPF; Picanço-Diniz, DLW; do Nascimento, JLM; Herculano, AM. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol Res 2009, 60, 212–220. [Google Scholar]
- Caballero, A; Martínez, R; Lloveras, V; Ratera, I; Vidal-Gancedo, J; Wurst, K; Tárraga, A; Molina, P; Veciana, J. Highly selective chromogenic and redox or fluorescent sensors of Hg2+ in aqueous environment based on 1,4-disubstituted azines. J Am Chem Soc 2005, 127, 15666–15667. [Google Scholar]
- Díez-Gil, C; Caballero, A; Ratera, I; Tárraga, A; Molina, P; Veciana, J. Naked-eye and selective detection of mercury (II) ions in mixed aqueous media using a cellulose-based support. Sensors 2007, 7, 3481–3488. [Google Scholar]
- Huang, J; Xu, Y; Qian, X. A rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: A NS2-containing receptor. J Org Chem 2009, 74, 2167–2170. [Google Scholar]
- Lu, H; Xiong, L; Liu, H; Yu, M; Shen, Z; Li, F; You, X. A highly selective and sensitive fluorescent turn-on sensor for Hg2+ and its application in live cell imaging. Org Biomol Chem 2009, 7, 2554–2558. [Google Scholar]
- Gong, J; Zhou, T; Song, D; Zhang, L; Hu, X. Stripping voltammetric detection of mercury(II) based on a bimetallic Au-Pt inorganic-Organic hybrid nanocomposite modified glassy carbon electrode. Anal Chem 2010, 82, 567–573. [Google Scholar]
- Loe-Mie, F; Marchand, G; Berthier, J; Sarrut, N; Pucheault, M; Blanchard-Desce, M; Vinet, F; Vaultier, M. Towards an efficient microsystem for the real-time detection and quantification of mercury in water based on a specifically designed fluorogenic binary task-specific ionic liquid. Angew Chem Int Ed 2010, 49, 424–427. [Google Scholar]
- Han, WS; Lee, HY; Jung, SH; Lee, SJ; Jung, JH. Silica-based chromogenic and fluorogenic hybrid chemosensor materials. Chem Soc Rev 2009, 38, 1904–1915. [Google Scholar]
- Nolan, EM; Lippard, SJ. Tools and tactics for the optical detection of mercuric ion. Chem Rev 2008, 108, 3443–3480. [Google Scholar]
- Selid, PD; Xu, H; Collins, EM; Face-Collins, MS; Zhao, JX. Sensing mercury for biomedical and environmental monitoring. Sensors 2009, 9, 5446–5459. [Google Scholar]
- Molina, P; Tárraga, A; Caballero, A. Ferrocene-based small molecules for multichannel molecular recognition of cations and anions. Eur J Inorg Chem 2008, 22, 3401–3417. [Google Scholar]
- Otón, F; Espinosa, A; Tárraga, A; Ratera, I; Wurst, K; Veciana, J; Molina, P. Mononuclear ferrocenophane structural motifs with two thiourea arms acting as a dual binding site for anions and cations. Inorg Chem 2009, 48, 1566–1576. [Google Scholar]
- Romero, T; Caballero, A; Espinosa, A; Tárraga, A; Molina, P. A multiresponsive two-arm ferrocene-based chemosensor molecule for selective detection of mercury. Dalton Trans 2009, 12, 2121–2129. [Google Scholar]
- Alfonso, M; Sola, A; Caballero, A; Tárraga, A; Molina, P. Heteroditopic ligands based on ferrocenyl benzimidazoles fused to an additional diaza heterocyclic ring system. Dalton Trans 2009, 43, 9653–9658. [Google Scholar]
- He, W; Myers, MR; Hanney, B; Spada, AP; Bilder, G; Galzcinski, H; Amin, D; Needle, S; Page, K; Jayyosi, Z; Perrone, MH. Potent quinoxaline-based inhibitors of PDGF receptor tyrosine kinase activity. Part 2: The synthesis and biological activities of RPR127963, an orally bioavailable inhibitor. Bioorg. Med. Chem. Lett 2003, 13, 3097–3100. [Google Scholar]
- Kim, YB; Kim, YH; Park, JY; Kim, SK. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg Med Chem Lett 2004, 14, 541–544. [Google Scholar]
- Yamamoto, T; Sugiyama, K; Kushida, T; Inoue, T; Kanbara, T. Preparation of new electron-accepting π-conjugated polyquinoxalines. Chemical and electrochemical reduction, electrically conducting properties and use in light-emiting diodes. J Am Chem Soc 1996, 118, 3930–3937. [Google Scholar]
- Yamamoto, T. π-Conjugated polymers with electronic and optical functionalities: Preparation by organometallic polycondensation, properties and applications. Macromol Rapid Commun 2002, 23, 583–606. [Google Scholar]
- Sessler, JL; Cho, D-G; Lynch, V. Diindolylquinoxalines: Effective indole-based receptors for phosphate anion. J Am Chem Soc 2006, 128, 16518–16519. [Google Scholar]
- Zapata, F; Caballero, A; Espinosa, A; Tárraga, A; Molina, P. A selective redox and chromogenic probe for Hg(II) in aqueous environment based on a ferrocene-azaquioxaline dyad. Inorg Chem 2009, 48, 11566–11575. [Google Scholar]
- Alfonso, M; Tárraga, A; Molina, P. Ferrocene-based multichannel molecular chemosensors with high selectivity and sensitivity for Pb(II) and Hg(II) metal cations. Dalton Trans 2010, 39, 8637–8645. [Google Scholar]
- Brown, DJ. The chemistry of heterocyclic compounds. In Quinoxalines: Supplement II; Taylor, EC, Wipf, P, Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Sanderson, CT; Quinlan, JA; Conover, RC; Johnson, MK; Murphy, M; Dluhy, RA; Kutal, C. Characterization of the low-energy electronic excited states of benzoyl-substituted ruthenocenes. Inorg Chem 2005, 44, 3283–3289. [Google Scholar]
- Barlow, S; Bunting, HE; Ringham, C; Green, JC; Bublitz, GU; Boxer, SG; Perry, JW; Marder, SR. Studies of the electronic structure of metallocene-based second-order nonlinear optical dyes. J Am Chem Soc 1999, 121, 3715–3723. [Google Scholar]
- Marder, SR; Perry, JW; Tiemann, BG; Schaefer, WP. Organometallic salts with large second-harmonic-generation powder efficiencies: (E)-1-ferrocenyl-2-(1-methyl-4-pyridiniumyl)ethylene salts. Organometallics 1991, 10, 1896–1901. [Google Scholar]
- Coe, BJ; Jones, CJ; McCleverty, JA; Bloor, D; Cross, G. An assessment of second harmonic generation by donor acceptor molecules containing stilbenyl or diarylazo bridges between ferrocenyl donor and nitro acceptor groups. J Organomet Chem 1994, 464, 225–232. [Google Scholar]
- Müller, TJ; Netz, A; Ansorge, M. Syntheses and NLO properties of chromium carbonyl arene complexes with conjugated side chains: The amphoteric nature of chromium carbonyl complexation in push-pull chromophores. Organometallics 1999, 18, 5066–5074. [Google Scholar]
- Carr, JD; Coles, SJ; Hassan, WW; Hursthouse, MB; Malik, KMA; Tucker, JHR. The effect of protonation on the spectroscopic and redox properties of a series of ferrocenoyl derivatives. J Chem Soc Dalton Trans 1999, 57–62. [Google Scholar]
- Specfit/32 Global Analysis System, 1999−2004, Spectrum Software Associates ([email protected]). The Specfit program was aquired from Bio-logic, SA ( www.bio-logic.info) in January 2005. The equation to be adjusted by nonlinear regression using the above-mentioned software was ΔA/b = {K11ΔɛHG[H]tot[G]}/{1 + K11[G]}, where H = host, G = guest, HG = complex, ΔA = variation in the absorption, b = cell width, K11 = association constant for a 1:1 model, and ΔɛHG = variation of molar absorptivity.
- Shortreed, M; Kopelman, R; Kuhn, M; Hoyland, B. Fluorescent fiber-optic calcium sensor for physiological measurements. Anal Chem 1996, 68, 1414–1418. [Google Scholar]





© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zapata, F.; Caballero, A.; Molina, P.; Tarraga, A. A Ferrocene-Quinoxaline Derivative as a Highly Selective Probe for Colorimetric and Redox Sensing of Toxic Mercury(II) Cations. Sensors 2010, 10, 11311-11321. https://doi.org/10.3390/s101211311
Zapata F, Caballero A, Molina P, Tarraga A. A Ferrocene-Quinoxaline Derivative as a Highly Selective Probe for Colorimetric and Redox Sensing of Toxic Mercury(II) Cations. Sensors. 2010; 10(12):11311-11321. https://doi.org/10.3390/s101211311
Chicago/Turabian StyleZapata, Fabiola, Antonio Caballero, Pedro Molina, and Alberto Tarraga. 2010. "A Ferrocene-Quinoxaline Derivative as a Highly Selective Probe for Colorimetric and Redox Sensing of Toxic Mercury(II) Cations" Sensors 10, no. 12: 11311-11321. https://doi.org/10.3390/s101211311



