Organic ISFET Based on Poly (3-hexylthiophene)
Abstract
:1. Introduction
2. Results and Discussion
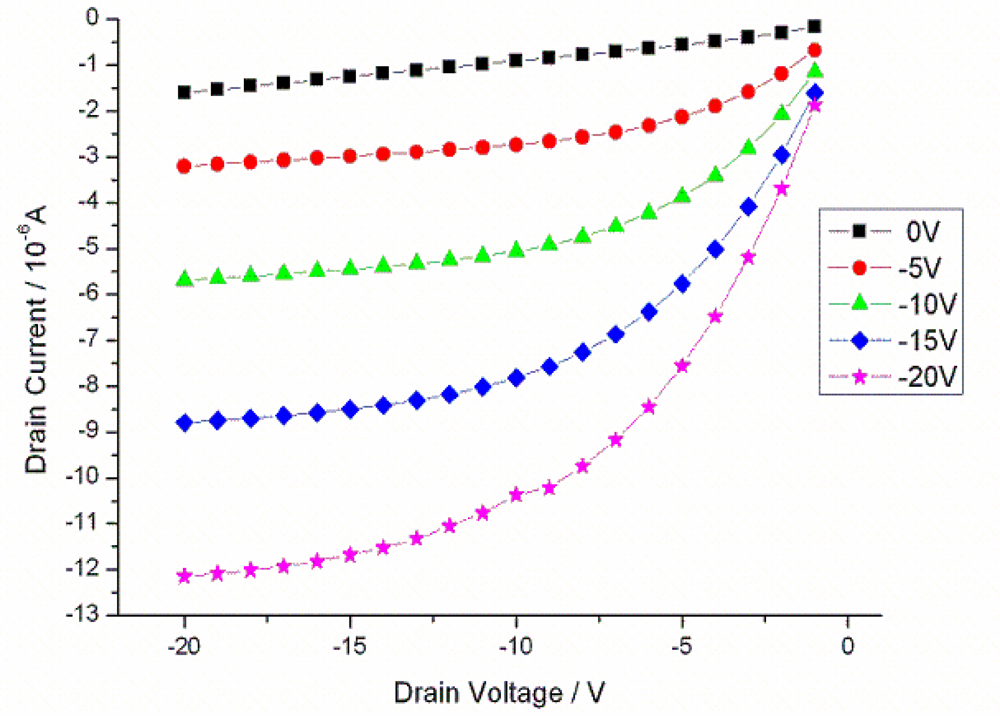
2.1. Measurement in Ambient Conditions and Thermal Behavior of Transistors in Liquid
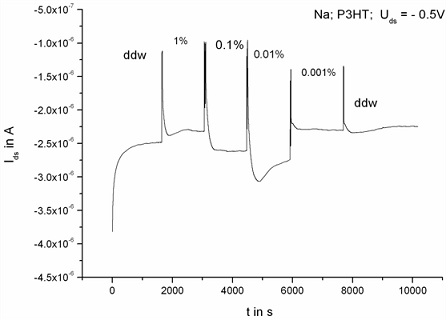
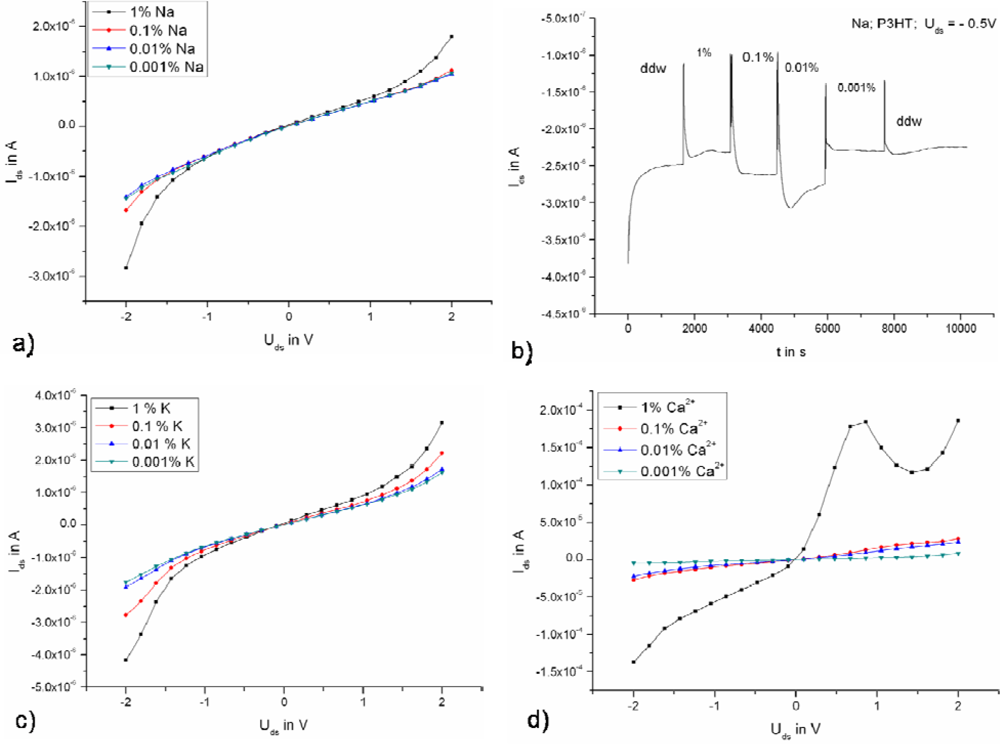
2.2. Ion sensitivity of the Transistors
2.3. pH Dependence
2.4. DC and AC Characterization
3. Experimental Section
3.1. Sample Holder
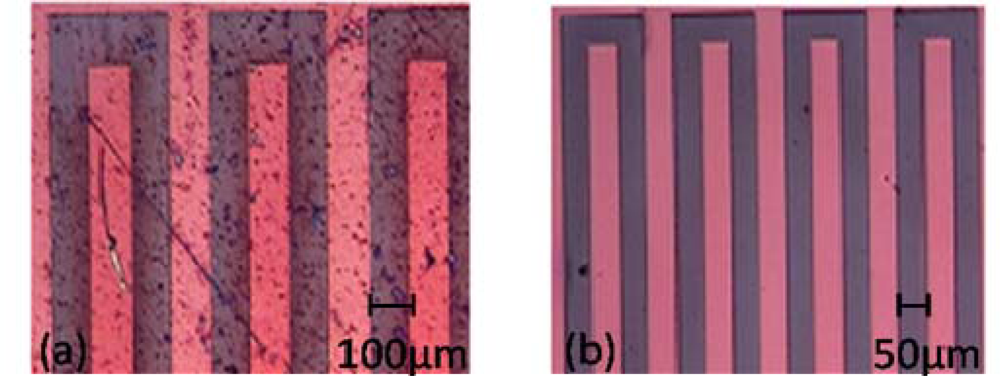
3.2. Fabrication of P3HT-Based Transistors
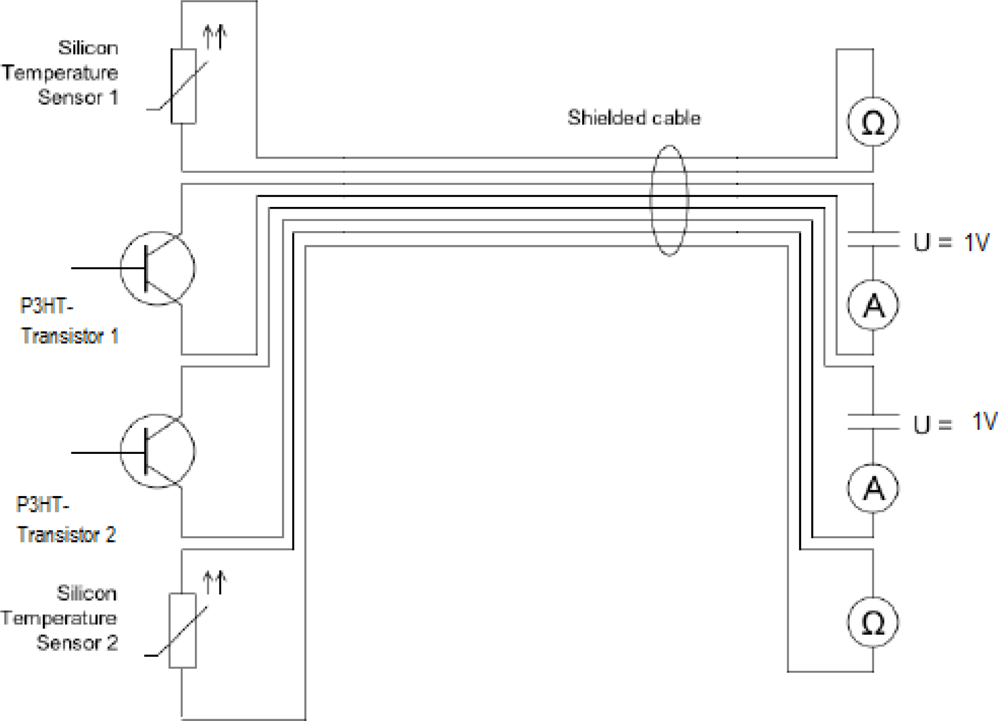
3.3. Experimental Set-Up
4. Conclusions
Acknowledgments
References and Notes
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysical Measurements. IEEE Trans. Biomed. Eng 1970, 17, 70–71. [Google Scholar]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuat. B 2003, 88, 1–20. [Google Scholar]
- Yuqing, M.; Jianguo, G.; Jianrong, C. Ion sensitive field effect transducer-based biosensors. Biotech. Adv 2003, 21, 527–534. [Google Scholar]
- Bartic, C.; Borghs, G. Organic thin-film transistors as transducers for (bio) analytical applications. Anal. Bioanal. Chem 2006, 384, 354–365. [Google Scholar]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater 2003, 2, 19–24. [Google Scholar]
- Mabeck, J.T.; Malliaras, G.G. Chemical and biological sensors based on organic thin-film transistors. Anal. Bioanal. Chem 2006, 384, 343–352. [Google Scholar]
- Bartic, C.; Campitelli, A.; Borghs, G. Field-effect detection of chemical species with hybrid organic/inorganic transistors. Appl. Phys. Lett 2003, 82, 475–477. [Google Scholar]
- Loi, A.; Manunza, I.; Bonfiglio, A. Flexible, organic, ion-sensitive field-effect transistor. Appl. Phys. Lett 2005, 86, 103512. [Google Scholar]
- Ji, T.; Rai, P.; Jung, S.; Varadan, V.K. In-vitro evaluation of flexible pH and potassium ion-sensitive organic field effect transistor sensors. Appl. Phys. Lett 2008, 92, 233304. [Google Scholar]
- Sargent, A.; Loi, T.; Gal, S.; Sadik, O.A. The electrochemistry of antibody-modified conducting polymer electrodes. J. Electroanal. Chem 1999, 470, 144–156. [Google Scholar]
- Sargent, A.; Sadik, O.A. Monitoring antibody-antigen reactions at conducting polymer-based immunosensors using impedance spectroscopy. Electrochim. Acta 1999, 44, 4667–4675. [Google Scholar]
- Meijerink, M.G.H.; Strike, D.J.; de Rooij, N.F.; Koudelka-Hep, M. Reproducible fabrication of an array of gas-sensitive chemo-resistors with commercially available polyaniline. Sens. Actuat. B: Chem 2000, 68, 331–334. [Google Scholar]
- Someya, T.; Dodabalapur, A.; Gelperin, A.; Katz, H.E.; Bao, Z. Integration and response of organic electronics with aqueous microfluidics. Langmuir 2002, 18, 5299–5302. [Google Scholar]
- Roberts, M.E.; Mannsfeld, S.C.B.; Queralto, N.; Reese, C.; Locklin, J.; Knoll, W.; Bao, Z. Water-Stable organic transistors and their application in chemical and biological sensors. Proc. Natl. Acad. Sci. USA 2008, 105, 12134–12139. [Google Scholar]
- Roberts, M.E.; Mannsfeld, S.C.B.; Stoltenberg, R.M.; Bao, Z. Flexible plastic transistor-based chemical sensors. Org. Elect 2009, 10, 377–383. [Google Scholar]
- Goetz, S.M.; Erlen, C.M.; Grothe, H.; Wolf, B.; Lugli, P.; Scarpa, G. Organic field effect transistors for biosensing applications. Org. Elect 2009, 10, 573–580. [Google Scholar]
- Scarpa, G.; Idzko, A.L.; Goetz, S.M.; Thalhammer, S. Biocompatibility studies of functionalized regioregular poly(3-hexylthiophene) layers for sensing applications. Macromol. Biosci. mabi. 200900412, in press..
- Hamadani, B.H.; Natelson, D. Temperature dependent contact resistances in high-quality polymer field-effect transistors. Appl. Phys. Lett 2004, 84, 443–445. [Google Scholar]
- Tanese, M.C.; Fine, D.; Dodabalapur, A.; Torsi, L. Interface and gate bias dependence responses of sensing organic thin-film transistors. Biosens. Bioelectron 2005, 21, 782–788. [Google Scholar]
- Torsi, L.; Farinola, G.M.; Marinelli, F.; Tanese, M.C.; Omar, O.H.; Valli, L.; Babudri, F.; Palmisano, F.; Zambonin, P.G.; Naso, F. A sensitivity-enhanced field-effect chiral sensor. Nature Mater 2008, 7, 412–417. [Google Scholar]
- Bartic, C.; Palan, B.; Campitelli, A.; Borghs, G. Monitoring pH with organic-based field-effect transistors. Sens. Actuat. B 2002, 83, 115–122. [Google Scholar]


| Solutions | AC / Time | DC / Time |
|---|---|---|
| Medium Complete | −− / 15 min | −−− / 5 min |
| Medium with Collagen | + / 30 min | |
| Medium with Fibronectin | + / 30 min | |
| PBS | +++ / 2 h | |
| Glucose | +++ / 12 h | |
| Penicillin | ++ / 6 h | −− / 4 h |
| FKS | −− / 15 min | −− / 15 min |
| Medium w/o Pen/FKS | ++ / 6 h | |
| L-Arginine with ddw | +++ / 1 h | |
| L-Arginine with PBS | −− / 15 min | |
| L-Glutamin with ddw | + / 4 h | |
| L-Glutamin with PBS | −− / 30 min | |
| NaHCO3 | +++ / 6 h | |
| Medium-long term | ++ / 12 h |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Scarpa, G.; Idzko, A.-L.; Yadav, A.; Thalhammer, S. Organic ISFET Based on Poly (3-hexylthiophene). Sensors 2010, 10, 2262-2273. https://doi.org/10.3390/s100302262
Scarpa G, Idzko A-L, Yadav A, Thalhammer S. Organic ISFET Based on Poly (3-hexylthiophene). Sensors. 2010; 10(3):2262-2273. https://doi.org/10.3390/s100302262
Chicago/Turabian StyleScarpa, Giuseppe, Anna-Lena Idzko, Anandi Yadav, and Stefan Thalhammer. 2010. "Organic ISFET Based on Poly (3-hexylthiophene)" Sensors 10, no. 3: 2262-2273. https://doi.org/10.3390/s100302262