The Assessment for Sensitivity of a NO2 Gas Sensor with ZnGa2O4/ZnO Core-Shell Nanowires—a Novel Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chemicals and Materials
2.2. Preparation of ZnGa2O4/ZnO Core-Shell Nanowire Based Sensors
2.3. Characterization of ZnGa2O4/ZnO Core-Shell Nanowires
2.4. Measurement of Gas Sensing Properties
3. Results and Discussion
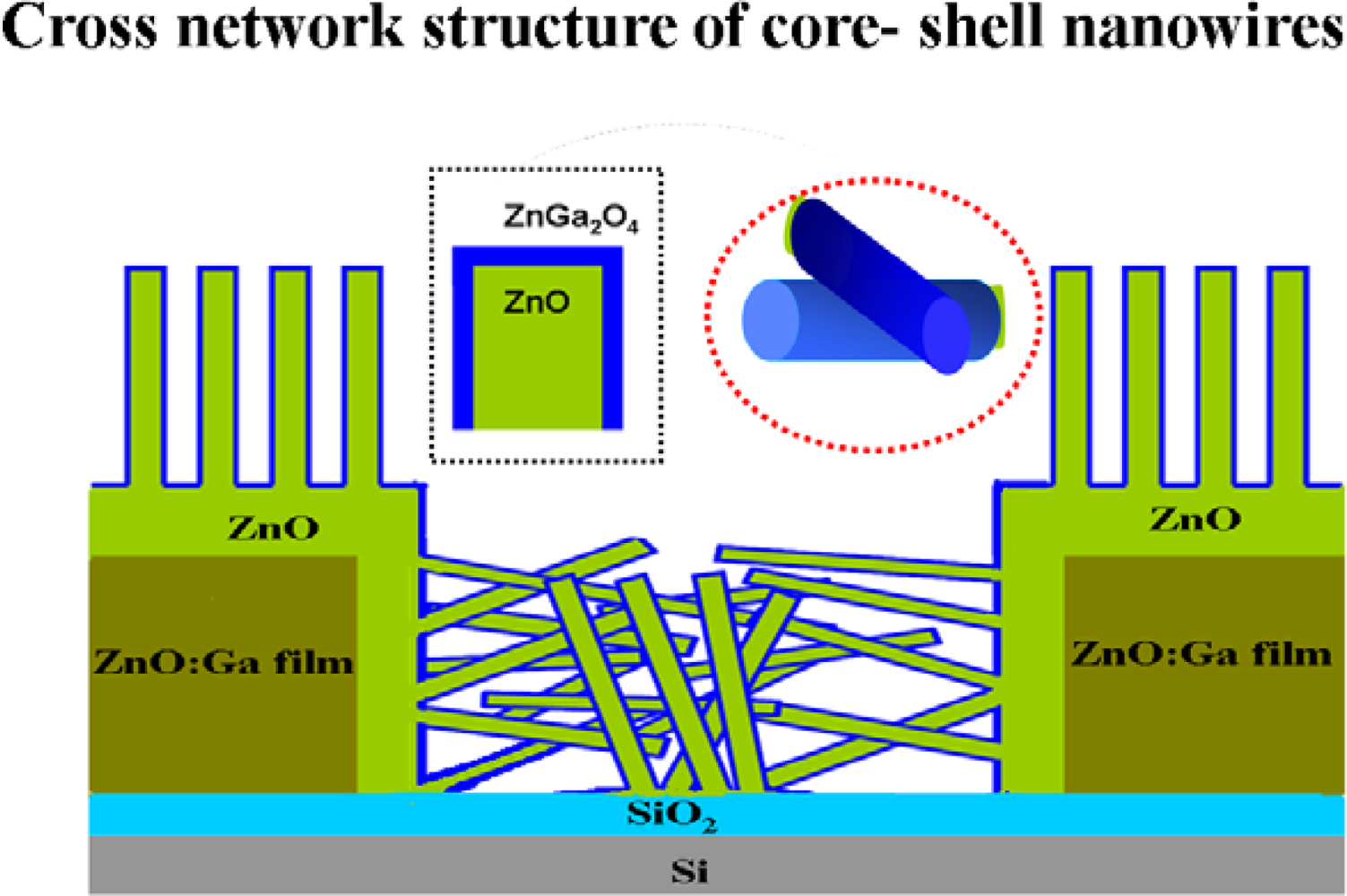
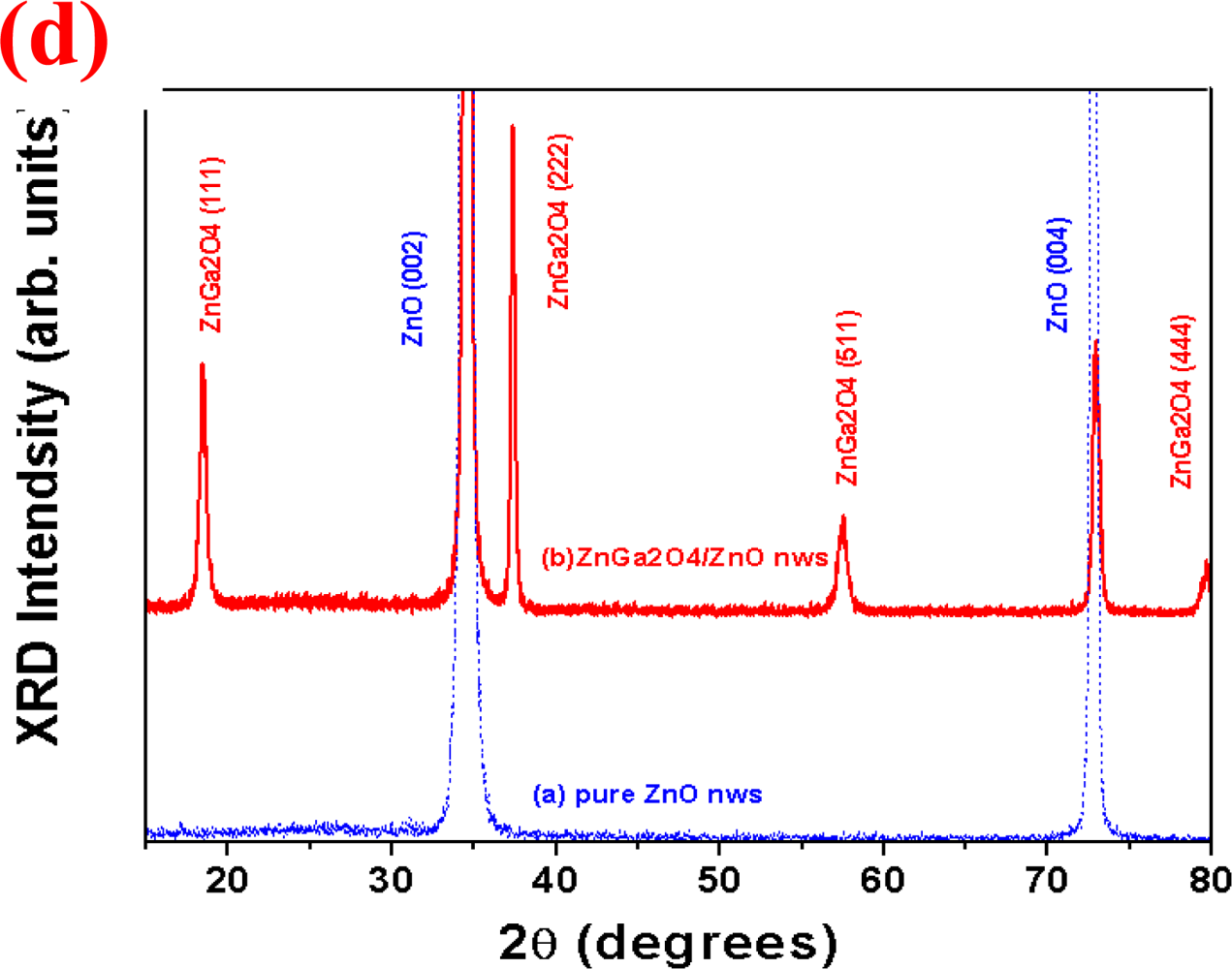
3.1. Morphological and Electronic Properties of ZnGa2O4/ZnO Core-Shell Nanowires
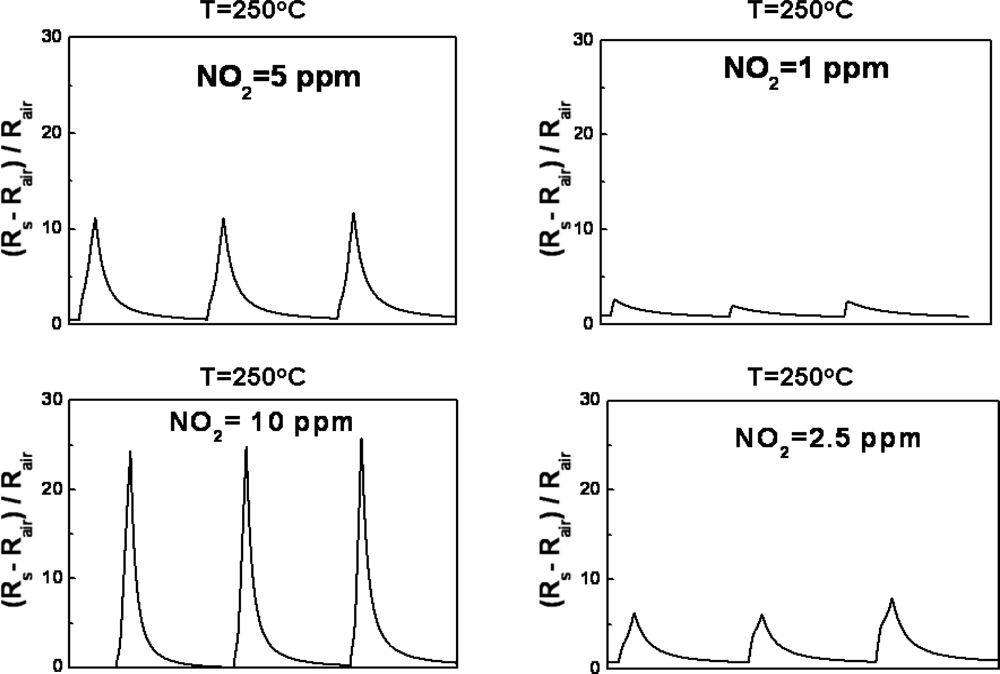
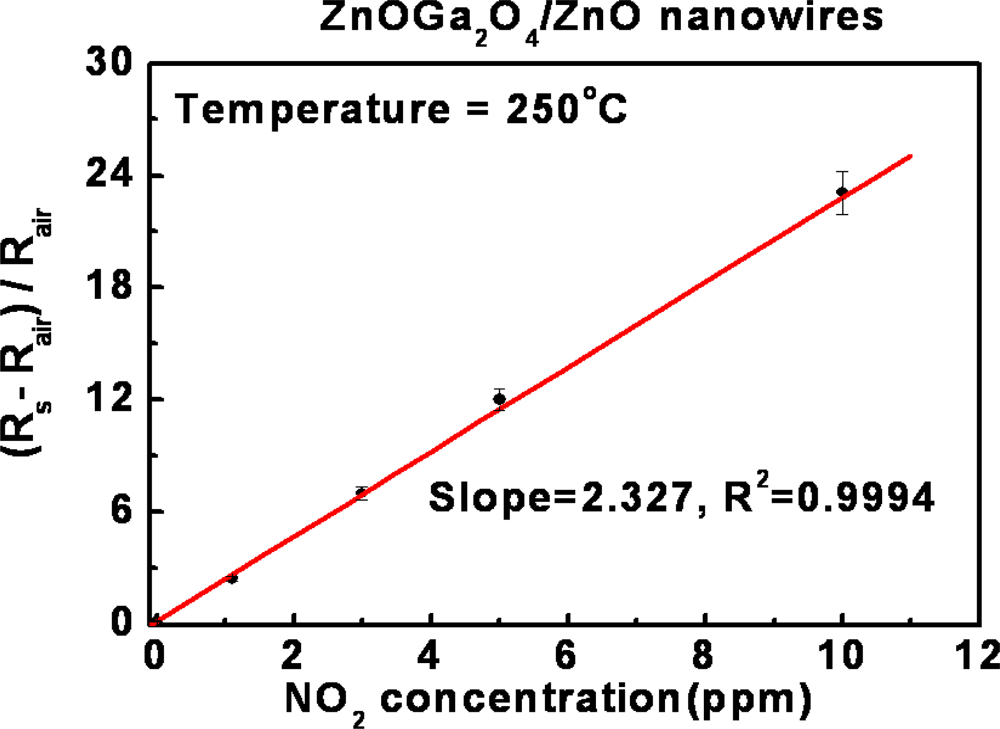
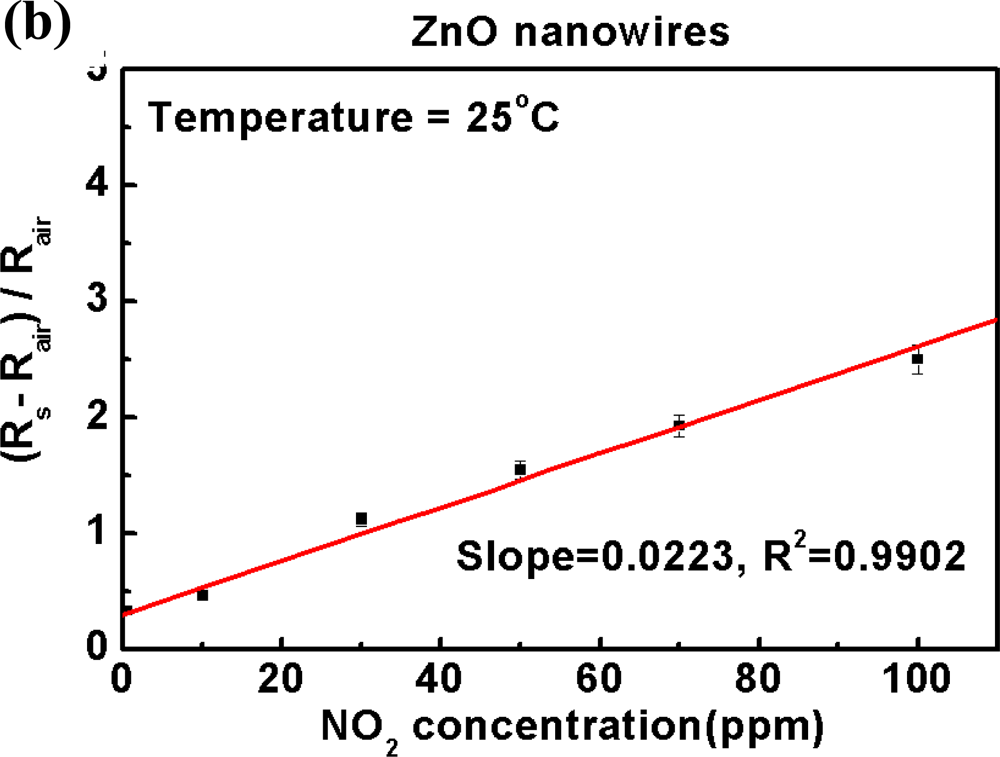
3.2. Efficacy and Sensitivity of Gas Detection of ZnGa2O4/ZnO Nanowires
4. Conclusions
Acknowledgments
References and Notes
- Guidotti, T.L. The higher oxides of nitrogen: inhalation toxicology. Environ. Res 1978, 15, 443–472. [Google Scholar]
- Richters, A.; Kuraitis, K. Inhalation of NO2 and blood borne cancer cell spread to the lungs. Arch. Environ. Health 1981, 36, 36–39. [Google Scholar]
- Baratto, C.; Sberveglieri, G.; Onischuk, A.; Caruso, B.; di Stasio, S. Low temperature selective NO2 sensors by nanostructured fibres of ZnO. Sens. Actuat. B: Chem 2004, 100, 261–265. [Google Scholar]
- Nenov, T.G.; Yordanov, S.P. Ceramic Sensors-Technology and Applications; Technomic Publishing: Lancaster, PA, USA, 1996. [Google Scholar]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuat. B:Chem 1996, 33, 198–202. [Google Scholar]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol 1997, 8, 223–237. [Google Scholar]
- Lu, G.; Miura, N.; Yamazoe, N. Stabilized zirconia-based sensors using WO3 electrode for detection of NO or NO2. Sens. Actuat. B: Chem 2000, 65, 125–127. [Google Scholar]
- Nakata, S.; Shimanoe, K.; Miura, N.; Yamazoe, N. Field effect transistor type NO2 sensor combine with NaNO2 auxiliary phase. Sens. Actuat. B: Chem 1999, 60, 49–56. [Google Scholar]
- Miura, N.; Ono, M.; Shimanoe, K.; Yamazoe, N. A compact solid-state amperometric sensor for detection of NO2 in ppb range. Sens. Actuat. B: Chem 1998, 49, 101–109. [Google Scholar]
- Ferro, R.; Rodriguez, J.A.; Bertrand, P. Development and characterization of a sprayed ZnO thin film-based NO2 sensor. Phys. Stat. Sol (c). 2005, 10, 3754–3757. [Google Scholar]
- Sergiu, T.; Shishiyanu, T.; Shishiyanu, S.; Lupan, O.I. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuat. B: Chem 2005, 107, 379–386. [Google Scholar]
- Steffes, H.; Imawan, C.; Solzbacher, F.; Obermeier, E. Enhancement of NO2 sensing properties of In2O3-based thin film using an Au or Ti surface modification. Sens. Actuat. B: Chem 2001, 78, 106–112. [Google Scholar]
- Tong, M.; Dai, G.; Wu, Y.; He, X.; Gao, D. WO3 thin film prepared by PECVD technique and its gas sensing properties to NO2. J. Mater. Sci 2001, 36, 2535–2538. [Google Scholar]
- Lee, D.S.; Lim, J.W.; Lee, S.M.; Huh, J.S.; Lee, D.D. Fabrication and characterization of micro-gas sensor for nitrogen oxide gas detection. Sens. Actuat. B: Chem 2000, 64, 31–36. [Google Scholar]
- Cantalini, C.; Sun, H.T.; Faccio, M.; Pelino, M.; Santucci, S.; Lozzi, L.; Passacantando, M. NO2 sensitivity of WO3 thin film obtained by highvacuum thermal evaporation. Sens. Actuat. B: Chem 1996, 60, 81–87. [Google Scholar]
- Lee, D.S.; Han, S.D.; Lee, S.M.; Huh, J.S.; Lee, D.D. The TiO2-adding effects in WO3-based NO2 sensors prepared by coprecipitation and precipitation method. Sens. Actuat. B: Chem 2000, 65, 331–335. [Google Scholar]
- Lee, D.S.; Han, S.D.; Huh, J.S.; Lee, D.D. Nitrogen oxides-sensing characteristics of WO3-based nanocrystalline thick film gas sensor. Sens. Actuat. B: Chem 1999, 60, 57–63. [Google Scholar]
- Chung, Y.K.; Kim, M.H.; Um, W.S.; Lee, H.S.; Song, J.K.; Choi, S.C.; Yi, K.M.; Lee, M.J.; Chung, K.W. Gas sensing properties of WO3 thick film for NO2 gas dependent on process condition. Sens. Actuat. B: Chem 1999, 60, 49–56. [Google Scholar]
- Giulio, M.D.; Micocci, G.; Serra, A.; Tepore, A. Sputter deposition of tungsten oxide for gas sensing applications. J. Mater. Sci 1998, 9, 317–322. [Google Scholar]
- Kumar, N.; Dorfman, A.; Hahm, J. Fabrication of optically enhanced ZnO nanorods and microrods using novel biocatalysts. J. Nanosci. Nanotechnol 2005, 5, 1915–1918. [Google Scholar]
- Hazra, S.K.; Basu, S. Hydrogen sensitivity of ZnO p–n homojunctions. Sens. Actuat. B: Chem 2006, 117, 177–182. [Google Scholar]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuat. B: Chem 2006, 115, 128–133. [Google Scholar]
- Wollenstein, J.; Plaza, J.A.; Cane, C.; Min, Y.; Bottner, H.; Tuller, H.L. A novel single chip thin film metal oxide array. Sens. Actuat. B: Chem 2003, 93, 350–355. [Google Scholar]
- Nakamura, Y.; Zhuang, H.; Kishimoto, A. Enhanced CO and CO2 gas sensitivity of the CuO/ZnO heterocontact made by quenched CuO ceramics. J. Electrochem. Soc 1998, 145, 632–638. [Google Scholar]
- Liu, C.Y.; Chen, C.F.; Leu, J.P. Fabrication and CO Sensing Properties of Mesostructured ZnO Gas Sensors. J. Electrochem. Soc 2009, 156, J16–J19. [Google Scholar]
- Hsueh, T.J.; Chen, Y.W.; Chang, S.J.; Wang, S.F.; Hsu, C.L.; Lin, Y. R.; Lin, T.S.; Chen, I.C. ZnO nanowire-based CO sensors prepared at various temperatures. J. Electrochem. Soc 2007, 154, J393–J396. [Google Scholar]
- Hsueh, T.J.; Chen, Y.W.; Chang, S.J.; Wang, S.F.; Hsu, C.L.; Lin, Y. R.; Lin, T.S.; Chen, I.C. ZnO nanowire-based CO sensors prepared on patterned ZnO:Ga/SiO2/Si templates. Sens. Actuat. B: Chem 2007, 125, 498–503. [Google Scholar]
- Hsueh, T.J.; Chang, S.J.; Lin, Y.R.; Chen, I.C.; Hsu, C.L. ZnO nanotube ethanol gas sensor. J. Electrochem. Soc 2008, 155, K152–K155. [Google Scholar]
- Heo, Y. W.; Varadarajan, V.; Kaufman, M.; Kim, K.; Norton, D.P.; Ren, F.; Fleming, P. H. Site-specific growth of ZnO nanorods using catalysis-driven molecular-beam epitaxy. Appl. Phys. Lett 2002, 81, 3046–3048. [Google Scholar]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Huang, H.; Lee, Y.C.; Tan, O.K.; Vayssieres, L. Hydrothermally grown oriented ZnO nanorod arrays for gas sensing applications. Nanotechnol 2006, 17, 4995–4998. [Google Scholar]
- Tseng, Y.K.; Hsu, H.C.; Hsieh, W.F.; Liu, K.S.; Chen, I.C. A Two-step Oxygen Injection Process for Growing ZnO Nanorods. J. Mater. Res 2003, 18, 2837–2844. [Google Scholar]
- Tseng, Y.K.; Huang, C.J.; Cheng, H.M.; Lin, I.N.; Liu, K.S.; Chen, I.C. Characterization and field emission properties of needle-like zinc oxide nanowhiskers grown vertically on conductive zinc oxide films. Adv. Funct. Mater 2003, 13, 811–814. [Google Scholar]
- Hsu, C.L.; Chang, S.J.; Hung, H. C.; Lin, Y.R.; Lu, T.H.; Tseng, Y.K.; Chen, I.C. Selective growth of vertical ZnO nanowires on ZnO:Ga/Si3N4/SiO2/Si templates. J. Vacuum Sci. and Technol. B: Microelectron. Nanometer Struct 2005, 23, 2292–2296. [Google Scholar]
- Hsu, C.L.; Chang, S.J.; Hung, H. C.; Lin, Y.R.; Lu, T.H.; Huang, C.J.; Tseng, Y.K.; Chen, I.C. Vertical single crystal ZnO nanowires grown on ZnO:Ga/glass templates. IEEE Trans. nanotechnol 2005, 4, 649–654. [Google Scholar]
- Hsu, C.L.; Chang, S.J.; Hung, H.C.; Tseng, Y.K.; Lin, Y.R.; Huang, C.J.; Chen, I.C. Well aligned vertically Al-doped ZnO nanowires synthesized on ZnO:Ga/glass templates”. J. Electrochem. Soc 2005, 152, G378–G381. [Google Scholar]
- Hsu, C.L.; Chang, S.J.; Lin, Y.R.; Tseng, Y.K.; Tsai, S.Y.; Chen, I.C. Vertically well aligned P-doped ZnO nanowires synthesized on ZnO:Ga/glass templates. Chem. Comm 2005, 28, 3571–3573. [Google Scholar]
- Hsu, C.L.; Lin, Y.R.; Chang, S.J.; Tseng, Y.K.; Tsai, S.Y.; Chen, I.C. Vertical ZnGa2O4/ZnO nanorods core shell grown on ZnO/glass template by reactive evaporation. Chem. Phys. Lett 2005, 411, 221–224. [Google Scholar]
- Hsu, C.L.; Lin, Y.R.; Chang, S.J.; Lu, T.H.; Lin, T.S.; Tsai, S.Y.; Chen, I.C. Influence of the formation of the second phase in ZnO/Ga nanowire systems. J. Electrochem. Soc 2006, 153, G333–G336. [Google Scholar]
- Yang, S.H. Electrophoretic Prepared ZnGa2O4 Phosphor Film for FED. J. Electrochem. Soc 2003, 150, H250–H253. [Google Scholar]
- Kang, S.W.; Jeon, B.S.; Yoo, J.S.; Lee, J.D. Optical characteristics of the phosphor screen in field-emission environments. J. Vac. Sci. Technol. B 1997, 15, 520–523. [Google Scholar]
- Kawazoe, H.; Ueda, K. Transparent conducting oxides based on the spinel structure. J. Am. Ceram. Soc 1999, 82, 3330–3336. [Google Scholar]
- Lee, Y.E.; Norton, D.P.; Budai, J.D.; Wei, Y. Enhanced ultraviolet photoconductivity in semiconducting ZnGa2O4 thin films. J. Appl. Phys 2001, 90, 3863–3866. [Google Scholar]
- Hsueh, T.J.; Chang, S.J.; Lin, Y. R.; Tsai, S.Y.; Chen, I.C.; Hsu, C.L. A Novel Method for the Formation of Ladder-like ZnO Nanowires. Cryst. Growth Des 2006, 6, 1282–1284. [Google Scholar]
- Mitra, P.; Chatterjee, A.P.; Maiti, H.S. ZnO thin film sensor. Mater. Lett 1998, 35, 33–38. [Google Scholar]
- Kohl, D. The role of noble metals in the chemistry of solid-state gas sensor. Sens. Actuat. B 1990, 1, 158–165. [Google Scholar]
- Sahay, P.P. Zinc oxide thin film gas sensor for detection of acetone. J. Mater. Sci 2005, 40, 4383–4385. [Google Scholar]
- Belysheva, T.V.; Bogovtseva, L.P.; Kazachkov, E.A.; Serebryakova, N.V. Gas- sensingproperties of doped In2O3 films as sensors for NO2 in air. J. Anal. Chem 2003, 58, 583–587. [Google Scholar]
- Ferro, R.; Rodriguez, J.A.; Bertrand, P. Peculiarities of nitrogen dioxide detection with sprayed undoped and indium-doped zinc oxide thin films. Thin Solid Films 2008, 516, 2225–2230. [Google Scholar]
- Sadek, A.Z.; Choopun, S.; Wlodarski, W.; Ippolito, S.J.; Kalantar-zadeh, K. Characterization of ZnO nanobelt-based gas sensor for H2, NO2, and hydrocarbon sensing. IEEE Sens. J 2007, 7, 919–924. [Google Scholar]






© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, I.-C.; Lin, S.-S.; Lin, T.-J.; Hsu, C.-L.; Hsueh, T.J.; Shieh, T.-Y. The Assessment for Sensitivity of a NO2 Gas Sensor with ZnGa2O4/ZnO Core-Shell Nanowires—a Novel Approach. Sensors 2010, 10, 3057-3072. https://doi.org/10.3390/s100403057
Chen I-C, Lin S-S, Lin T-J, Hsu C-L, Hsueh TJ, Shieh T-Y. The Assessment for Sensitivity of a NO2 Gas Sensor with ZnGa2O4/ZnO Core-Shell Nanowires—a Novel Approach. Sensors. 2010; 10(4):3057-3072. https://doi.org/10.3390/s100403057
Chicago/Turabian StyleChen, I-Cherng, Shiu-Shiung Lin, Tsao-Jen Lin, Cheng-Liang Hsu, Ting Jen Hsueh, and Tien-Yu Shieh. 2010. "The Assessment for Sensitivity of a NO2 Gas Sensor with ZnGa2O4/ZnO Core-Shell Nanowires—a Novel Approach" Sensors 10, no. 4: 3057-3072. https://doi.org/10.3390/s100403057



