Corrosion Sensor for Monitoring the Service Condition of Chloride-Contaminated Cement Mortar
Abstract
:1. Introduction
2. Experimental
2.1. Materials
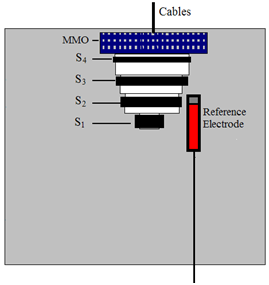
2.2. Sensor Arrangements
- Each steel anode has an equal exposure area of about 668 mm2. The surface area of the cathode (more than 3,000 mm2) is sufficiently larger than the anodes to reduce errors due to the lateral current distribution on the surface of the steel anodes [21].
- The cement mortar cover over each steel anode is not affected by the parts of the sensor, thus, the penetration of chloride into each anode and the carbonation of the surrounding cement mortar are not influenced by the adjacent anodes.
- The sensors can be used in existing concrete structures by inserting them into holes drilled in cover-zone concrete. Furthermore, all of the measured parameters are depth-related. In this way, the penetration of chloride and/or carbonation from the cement mortar surface into the cement mortar and the subsequent corrosion risk of the reinforcing structures can be measured immediately.
2.3. Specimens and Storage Conditions
2.3.1. Mixture proportions of the cement mortar
2.3.2. Short-term wetting conditions
2.4. Measurement Theory and Procedure
2.4.1. Electrochemical impedance spectroscopy (EIS)
2.4.2. Linear polarization resistance
3. Results and Discussion
3.1. Cover-zone Cement Mortar Resistance
3.2. Polarization Resistance Measured by the LPR Method
4. Conclusions
Acknowledgments
References and Notes
- Raupach, M. Chloride-induce Macrocell Corrosion of Steel in Concrete—Theoretical Background and Practical Consequences. Cons. Mater 1996, 10, 329–338. [Google Scholar]
- Pech-Canul, M.A.; Castro, P. Corrosion Measurement of Steel Reinforcement in Concrete Exposed to a Tropical marine atmosphere. Cem. Concr. Res 2002, 32, 491–498. [Google Scholar]
- Azevedo, C.R.F.; Marques, E.R. Three-dimensional Analysis of Fracture, Corrosion and Wear Surface. Eng. Fail. Anal 2010, 17, 286–300. [Google Scholar]
- Ahmad, S. Reinforcement Corrosion in Concrete Structures, Its Monitoring and Service Life Prediction—A Review. Cem. Con. Comp 2003, 25, 459–471. [Google Scholar]
- Cao, J.Y.; Chung, D.D.L. Electric Polarization and Depolarization in Cement-based Materials, Studied by Apparent Electrical Resistance. Cem. Concr. Res 2004, 34, 481–485. [Google Scholar]
- Keddam, M.; Takenouti, H.; Novoa, X.R.; Andrade, C.; Alonso, C. Impedance Measurements on Cement Paste. Cem. Concr. Res 1997, 27, 1191–1201. [Google Scholar]
- Newlands, M.D.; Jones, M.R.; Kandasami, S.; Harrison, T.A. Sensitivity of Electrode Contact Solutions and Contact Pressure in Assessing Electrical Resistivity of Concrete. Mater. Struct 2007, 41, 621–632. [Google Scholar]
- McCarter, W.J.; Chrisp, T.M.; Starrs, G.; Basheer, P.A.M.; Blewett, J. Field Monitoring of Electrical Conductivity of Cover-zone Concrete. Cem. Con. Coms 2005, 27, 809–817. [Google Scholar]
- Polder, R.B. Test Method for on Site Measurement of Resistivity of Concrete—A RILEM TC-154 Technical Recommendation. Cons. Mater 2001, 15, 125–131. [Google Scholar]
- Raupach, M.; Schieβl, P. Macrocell Sensor System for Monitoring of the Corrosion Risk of the Reinforcement in Concrete Strucures. NDT&E Int 2001, 34, 435–442. [Google Scholar]
- Millard, S.G.; Law, D.; Bungey, J.H.; Cairns, J. Environmental Influences on Linear Polarisation Corrosion Rate Measurement in Reinforced Concrete. NDT&E Int 2001, 34, 409–417. [Google Scholar]
- Wojtas, H. Determination of Corrosion Rate of Reinforcement with a Modulated Guard Ring Electrode; Analysis of Errors Due to Lateral Current Distribution. Corros. Sci 2004, 46, 1621–1632. [Google Scholar]
- Liu, Y.P.; Weyers, R.E. Comparison of Guarded and Unguard Linear Polarization CCD Devices with Weight Loss Measurements. Cem. Concr. Res 2003, 33, 1093–1101. [Google Scholar]
- Andrade, C.; Martinez, I. Calibration by Gravimetric Losses of Electrochemical Corrosion Rate Measurement Using Modulated Confinement of the Current. Mater. Struct 2005, 38, 833–841. [Google Scholar]
- Bohni, H. Corrosion in Reinforced Concrete Structures, 1st ed.; Ma, H., Ed.; Mechanical Industry Press: Beijing, China, 2009; pp. 40–59. [Google Scholar]
- Gu, P.; Arsenault, B.; Beaudoin, J.J.; Legoux, J.G.; Harvey, B.; Fournier, J. Polarization Resistance of Stainless Steel-coated Rebars. Cem. Concr. Res 1998, 28, 321–327. [Google Scholar]
- Basheer, P.A.M.; Gilleece, P.R.V.; Long, A.E.; McCarter, W.J. Monitoring Electrical Resistance of Concretes Containing Alternative Cementitious Materials to Assess Their Resistance to Chloride Penetration. Cem. Con. Coms 2002, 24, 437–449. [Google Scholar]
- Chung, D.D.L. Damage in Cement-based Materials, Studied by Electrical Resistance Measurement. Mat. Sci. Eng. R 2003, 42, 1–40. [Google Scholar]
- Lu, S.; Wang, Z.; Ba, H.J.; Yang, Y.Z. Preparation of Ti/MnO2 Reference Electrode and Its Application in Concrete Structures. J. Wuhan Univ. Technol.-Mater. Sci. Ed 2009, 89, 161–165. [Google Scholar]
- Lu, S.; Ba, H.J.; Yang, Y.Z. Monitoring Depth-related Electrical Resistivity of Coverzone Concrete by Tower Type Sensor Using Method of AC Impedance. Proceedings of the IEEE International Conference on Test and Measurement 2009, HongKong, China, December 2009.
- Castro, P.; Veleva, L.; Balancan, M. Corrosion of Reinforced Concrete in a Tropical Marine Environment and in Accelerated Tests. Cons. Mater 1997, 11, 75–81. [Google Scholar]
- Morris, W.; Vico, A.; Vazquez, M.; de Sanchez, S.R. Corrosion of Reinforcing Steel Evaluated By Means of Concrete Resistivity Measurements. Corros. Sci 2002, 44, 81–99. [Google Scholar]
- Sagues, A.A.; Kranc, S.C.; Moreno, E.I. Evaluation of Electrochemical Impedance With Constant Phase Angle Component From the Galvanostatic Step Response of Steel in Concrete. Electrochim. Acta 1996, 41, 1239–1243. [Google Scholar]
- Cao, C.N.; Zhang, J.Q. Electrochemical Impedance Spectroscopy Introductory Theory, 1st ed.; Scientific Press: Beijing, China, 2002; pp. 23–31. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1996; pp. 472–475. [Google Scholar]
- Lius, T.; Weyers, R.W. Modeling the Dynamic Corrosion Process in Chloride Contaminated Concrete Structures. Cem. Concr. Res 1998, 28, 365–379. [Google Scholar]
- Alonso, C.; Andrade, C.; Gonzalez, J.A. Relation between Resistivity and Corrosion Rate of Reinforcements in Carbonated Mortar Made with Several Cement Types. Cem. Concr. Res 1988, 18, 687–698. [Google Scholar]
- Correia, M.J.; Pereira, E.V.; Salta, M.M.; Fonseca, I.T.E. Sensor for Oxygen Evaluation in Concrete. Cem. Con. Coms 2006, 28, 226–232. [Google Scholar]
- Hunkeler, F. The Resistivity of Pore Water Solution—A Decesive Parameter of Rebar Corrosion and Repair Methods. Cons. Mater 1996, 10, 381–389. [Google Scholar]
- Ong, K.G.; Paulose, M.; Jain, M.K.; Gong, D.; Varghese, O.K.; Mungle, C.; Grimes, C.A. Magnetism-Based Remote Query Glucose Sensors. Sensors 2001, 1, 138–147. [Google Scholar]
- Dickey, E.C.; Varghese, O.K.; Ong, K.G.; Gong, D.; Paulose, M.; Grimes, C.A. Room Temperature Ammonia and Humidity Sensing Using Highly Ordered Nanoporous Alumina Films. Sensors 2002, 2, 91–110. [Google Scholar]







| Elements in % | C | Mn | P | S | Si | Fe |
|---|---|---|---|---|---|---|
| Steel anode | 0.181 | 0.580 | 0.012 | 0.023 | 0.350 | 97.5 |
| Anode | Inside diameter | Outside diameter | Depth |
|---|---|---|---|
| S1 | 8.0 | 24.0 | 8.7 |
| S2 | 24.0 | 33.0 | 7.7 |
| S3 | 33.0 | 40.0 | 6.7 |
| S4 | 40.0 | 46.0 | 6.0 |
| Mix | Cement | Fine agg. | Water | NaCl (%) |
|---|---|---|---|---|
| A | 1 | 3 | 0.40 | 0 |
| B | 1 | 3 | 0.40 | 1.0 |
| C | 1 | 3 | 0.40 | 3.0 |
| D | 1 | 3 | 0.40 | 5.0 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, S.; Ba, H.-J. Corrosion Sensor for Monitoring the Service Condition of Chloride-Contaminated Cement Mortar. Sensors 2010, 10, 4145-4158. https://doi.org/10.3390/s100404145
Lu S, Ba H-J. Corrosion Sensor for Monitoring the Service Condition of Chloride-Contaminated Cement Mortar. Sensors. 2010; 10(4):4145-4158. https://doi.org/10.3390/s100404145
Chicago/Turabian StyleLu, Shuang, and Heng-Jing Ba. 2010. "Corrosion Sensor for Monitoring the Service Condition of Chloride-Contaminated Cement Mortar" Sensors 10, no. 4: 4145-4158. https://doi.org/10.3390/s100404145