CMOS-Integrated Film Bulk Acoustic Resonators for Label-Free Biosensing
Abstract
:1. Introduction
2. Experimental Section
2.1. CMOS-Integrated FBAR Array
2.2. Chemicals
2.3. BSA Measurements
2.4. Functionalisation of the Gold Surfaces
3. Results and Discussion
3.1. Mass Sensitivity Comparison Obtained with FBAR, SPR and QCM
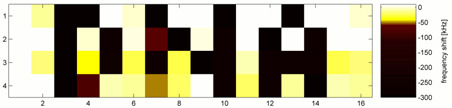
3.2. Multiplexed DNA Measurement
3.3. Multiplexed Measurement of PCR Amplified Products in Buffer and Serum
4. Conclusions
References and Notes
- Zimmermann, B.; Hahnefeld, C.; Herberg, F.W. Applications of biomolecular interaction analysis in drug development. TARGETS 2002, 1, 66–73. [Google Scholar]
- Gronewold, T.M.A. Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta 2007, 603, 119–128. [Google Scholar]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron 2007, 23, 151–160. [Google Scholar]
- Voinova, M.V.; Jonson, M.; Kasemo, B. Missing mass effect in biosensor's QCM applications. Biosens. Bioelectron 2002, 17, 835–841. [Google Scholar]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; Kasemo, B. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloid Surface B 2002, 24, 155–170. [Google Scholar]
- Grate, J.W.; Martin, S.J.; White, R.M. Acoustic wave microsensors. Anal. Chem 2008, 65, 940A–948A. [Google Scholar]
- Do, T.; Ho, F.; Heidecker, B.; Witte, K.; Chang, L.; Lerner, L. A rapid method for determining dynamic binding capacity of resins for the purification of proteins. Protein Express. Purif 2008, 60, 147–150. [Google Scholar]
- Concepcion, J.; Witte, K.; Wartchow, C.; Choo, S.; Yao, D.; Persson, H.; Wei, J.; Li, P.; Heidecker, B.; Ma, W.; Varma, R.; Zhao, L.S.; Perillat, D.; Carricato, G.; Recknor, M.; Du, K.; Ho, H.; Ellis, T.; Gamez, J.; Howes, M.; Phi-Wilson, J.; Lockard, S.; Zuk, R.; Tan, H. Label-Free Detection of Biomolecular Interactions Using BioLayer Interferometry for Kinetic Characterization. Comb. Chem. High Throughput Scr 2009, 12, 791–800. [Google Scholar]
- Arwin, H. Is ellipsometry suitable for sensor applications? Sensor. Actuat. A: Phys 2001, 92, 43–51. [Google Scholar]
- Drafts, B. Acoustic wave technology sensors. Sens. Mag 2000, 17. [Google Scholar]
- Carrascosa, L.G.; Moreno, M.; Álvarez, M.; Lechuga, L.M. Nanomechanical biosensors: a new sensing tool. Trac-Trend Anal. Chem 2006, 25, 196–206. [Google Scholar]
- Dickherber, A.; Corso, C.D.; Hunt, W. Lateral field excitation (LFE) of thickness shear mode (TSM) acoustic waves in thin film bulk acoustic resonators (FBAR) as a potential biosensor. Conf. Proc. IEEE Eng. Med. Biol. Soc 2006, 1, 4590–4593. [Google Scholar]
- Gabl, R.; Feucht, H.D.; Zeininger, H.; Eckstein, G.; Schreiter, M.; Primig, R.; Pitzer, D.; Wersing, W. First results on label-free detection of DNA and protein molecules using a novel integrated sensor technology based on gravimetric detection principles. Biosens. Bioelectron 2004, 19, 615–620. [Google Scholar]
- Bjurstrom, J.; Wingqvist, G.; Yantchev, V.; Katardjiev, I. 3I–5 Design and Fabrication of Temperature Compensated Liquid FBAR Sensors. Proc. IEEE Ultrason. Symp 2006, 898–901. [Google Scholar]
- Yanagitani, T.; Kiuchi, M.; Matsukawa, M.; Watanabe, Y. Characteristics of pure-shear mode BAW resonators consisting of (1120) textured ZnO films. IEEE Trans. Ultrason Ferroelectr. Freq. Control 2007, 54, 1680–1686. [Google Scholar]
- Akiyama, M.; Nagao, K.; Ueno, N.; Tateyama, H.; Yamada, T. Influence of metal electrodes on crystal orientation of aluminum nitride thin films. Vacuum 2004, 74, 699–703. [Google Scholar]
- Wang, J.S.; Lakin, K.M. Sputtered C-Axis Inclined ZnO Films for Shear Wave Resonators. IEEE T. Ultrason. Ferroelectr 1982, 480–483. [Google Scholar]
- Faiss, S.; Kastl, K.; Janshoff, A.; Steinem, C. Formation of irreversibly bound annexin A1 protein domains on POPC/POPS solid supported membranes. Biochim. Biophys. Acta 2008, 1778, 1601–1610. [Google Scholar]
- Martin, F.; Jan, M.E.; Rey-Mermet, S.; Su, D.; Muralt, P.; Cantoni, M. Shear mode coupling and tilted grain growth of A1N thin films in BAW resonators. Proc. IEEE Ultrason. Symp 2005, 333–336. [Google Scholar]
- Wingqvist, G.; Bjurstrom, J.; Liljeholm, L.; Katardjiev, I.; Spetz, A.L. Shear mode AlN thin film electroacoustic resonator for biosensor applications. Proc. IEEE Sensor 2005, 4. [Google Scholar]
- Link, M.; Schreiter, M.; Weber, J.; Pitzer, D.; Primig, R.; Assouar, M.B.; Elmazria, O. C-axis inclined ZnO films deposited by reactive sputtering using an additional blind for shear BAW devices. IEEE Ultrason. Symp 2005, 1, 202–205. [Google Scholar]
- Fardeheb-Mammeri, A.; Assouar, M.B.; Elmazria, O.; Gatel, C.; Fundenberger, J.J.; Benyoucef, B. C-Axis inclined AlN film growth in planar system for shear wave devices. Diam. Relat. Mater 2008, 17, 1770–1774. [Google Scholar]
- Nirschl, M.; Blüher, A.; Erler, C.; Katzschner, B.; Vikholm-Lundin, I.; Auer, S.; Vörös, J.; Pompe, W.; Schreiter, M.; Mertig, M. Film bulk acoustic resonators for DNA and protein detection and investigation of in vitro bacterial S-layer formation. Sens. Actuat. A: Physical 2009, 156, 180–184. [Google Scholar]
- Eichelbaum, F.; Borngraber, R.; Schroder, J.; Lucklum, R.; Hauptmann, P. Interface circuits for quartz-crystal-microbalance sensors. Rev. Sci. Instr 1999, 70, 2537–2545. [Google Scholar]
- Norling, M.; Enlund, J.; Katardjiev, I.; Gevorgian, S. Oscillators based on monolithically integrated AlN TFBARs. IEEE. Trans. Microwave Theory 2008, 56, 3209–3216. [Google Scholar]
- Augustyniak, M.; Weber, W.; Beer, G.; Mulatz, H.; Elbrecht, L.; Timme, H.J.; Tiebout, M.; Simburger, W.; Paulus, C.; Eversmann, B.; Schmitt-Landsiedel, D.; Thewes, R.; Brederlow, R. IEEE International Solid-State Circuits Conference (ISSCC 2007). Proceedings of An Integrated Gravimetric FBAR Circuit for Operation in Liquids Using a Flip-Chip Extended 0.13¬øm CMOS Technology, San Francisco, CA, USA, January 1, 2007; 2007; pp. 392–610. [Google Scholar]
- Schneider, T.; Doerner, S.; Hauptmann, P.R.; Richter, D.; Fritze, H. In Fast impedance analyzer interface with direct-sampling-technique for highly damped resonant gas sensors. Sensors IEEE 2005, 4, 361–368. [Google Scholar]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys J 1998, 75, 1397–1402. [Google Scholar]
- YuM, L.; Sukhorukov, G.B. Protein architecture: assembly of ordered films by means of alternated adsorption of oppositely charged macromolecules. Membr. Cell. Biol 1997, 11, 277–303. [Google Scholar]
- Link, M.; Schreiter, M.; Weber, J.; Gabl, R.; Pitzer, D.; Primig, R.; Wersing, W.; Assouar, M.B.; Elmazria, O. C-Axis inclined ZnO films for shear-wave transducers deposited by reactive sputtering using an additional blind. J. Vac. Sci. Technol. A 2006, 24, 218–222. [Google Scholar]
- Tukkiniemi, K.; Rantala, A.; Nirschl, M.; Pitzer, D.; Huber, T.; Schreiter, M. Fully integrated FBAR sensor matrix for mass detection. Proced. Chem 2009, 1, 1051–1054. [Google Scholar]
- Tappura, K.; Vikholm-Lundin, I.; Albers, W.M. Lipoate-based imprinted self-assembled molecular thin films for biosensor applications. Biosens. Bioelectron 2007, 22, 912–919. [Google Scholar]
- Vikholm-Lundin, I.; Auer, S.; Munter, T.; Fiegl, H.; Apostolidou, S. Hybridization of binary monolayers of single stranded oligonucleotides and short blocking molecules. Surf. Sci 2009, 603, 620–624. [Google Scholar]
- Stenberg, E.; Persson, B.; Roos, H.; Urbaniczky, C. Quantitative determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins. J. Colloid Inter. Sci 1991, 143, 513–526. [Google Scholar]
- Reimhult, E.; Larsson, C.; Kasemo, B.; Höök, F. Simultaneous Surface Plasmon Resonance and Quartz Crystal Microbalance with Dissipation Monitoring Measurements of Biomolecular Adsorption Events Involving Structural Transformations and Variations in Coupled Water. Anal. Chem 2004, 76, 7211–7220. [Google Scholar]
- Vig, J.R. On acoustic sensor sensitivity. Ultrasonics, Ferroelectrics and Frequency Control. IEEE Trans 1991, 38, 311. [Google Scholar]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. A Hadron. Nuclei 1959, 155, 206–222. [Google Scholar]
- Larsson, C.; Rodahl, M.; Hook, F. Characterization of DNA Immobilization and Subsequent Hybridization on a 2D Arrangement of Streptavidin on a Biotin-Modified Lipid Bilayer Supported on SiO2. J. Anal. Chem 2003, 75, 5080–5087. [Google Scholar]

 ), CMOS-integrated FBAR (
), CMOS-integrated FBAR (
 ) and SPR (□). The curve for the FBAR measurement is the average of 10 pixels, the SPR represents 4 channels; the error bars show the standard deviation.
) and SPR (□). The curve for the FBAR measurement is the average of 10 pixels, the SPR represents 4 channels; the error bars show the standard deviation.
 ), CMOS-integrated FBAR (
), CMOS-integrated FBAR (
 ) and SPR (□). The curve for the FBAR measurement is the average of 10 pixels, the SPR represents 4 channels; the error bars show the standard deviation.
) and SPR (□). The curve for the FBAR measurement is the average of 10 pixels, the SPR represents 4 channels; the error bars show the standard deviation.



 ) and serum (
) and serum (
 ) (1:100) with a surface layer of S-S-CALCA/Lipa-DEA. The error bars show the standard deviation over 4 pixels. All points were recorded on the same chip.
) (1:100) with a surface layer of S-S-CALCA/Lipa-DEA. The error bars show the standard deviation over 4 pixels. All points were recorded on the same chip.
 ) and serum (
) and serum (
 ) (1:100) with a surface layer of S-S-CALCA/Lipa-DEA. The error bars show the standard deviation over 4 pixels. All points were recorded on the same chip.
) (1:100) with a surface layer of S-S-CALCA/Lipa-DEA. The error bars show the standard deviation over 4 pixels. All points were recorded on the same chip.
| SPR | QCM | Passive FBAR | CMOS-integrated FBAR (best/average) | |
|---|---|---|---|---|
| Mass sensitivity | 10 RU cm2/ng [34] | 61.1 ppb cm2/ng (measured) 22.7 ppb cm2/ng (Sauerbrey [37]) | 5.63 ppm cm2/ng | 7.13 ppm cm2/ng |
| Noise level (3σ) | 0.63 RU | 23 ppb | 2.3 ppm | 3.0/10.8 ppm |
| Mass resolution (LOD) | 0.06 ng/cm2 | 0.38 ng/cm2 | 0.41 ng/cm2 | 0.42/1.5 ng/cm2 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nirschl, M.; Rantala, A.; Tukkiniemi, K.; Auer, S.; Hellgren, A.-C.; Pitzer, D.; Schreiter, M.; Vikholm-Lundin, I. CMOS-Integrated Film Bulk Acoustic Resonators for Label-Free Biosensing. Sensors 2010, 10, 4180-4193. https://doi.org/10.3390/s100504180
Nirschl M, Rantala A, Tukkiniemi K, Auer S, Hellgren A-C, Pitzer D, Schreiter M, Vikholm-Lundin I. CMOS-Integrated Film Bulk Acoustic Resonators for Label-Free Biosensing. Sensors. 2010; 10(5):4180-4193. https://doi.org/10.3390/s100504180
Chicago/Turabian StyleNirschl, Martin, Arto Rantala, Kari Tukkiniemi, Sanna Auer, Ann-Charlotte Hellgren, Dana Pitzer, Matthias Schreiter, and Inger Vikholm-Lundin. 2010. "CMOS-Integrated Film Bulk Acoustic Resonators for Label-Free Biosensing" Sensors 10, no. 5: 4180-4193. https://doi.org/10.3390/s100504180