Windows on the Human Body – in Vivo High-Field Magnetic Resonance Research and Applications in Medicine and Psychology
Abstract
:1. Introduction
Morphological MRI—Techniques and Applications
Advanced Morphological Imaging of Joints and Cartilage
Functional MRI—Techniques and Applications
Multi-Modal Integration at High Magnetic Field: MRI, fMRI and Slow Cortical Potentials (SCP)
Advanced Functional 23Na-Imaging of Articular Cartilage
In Vivo Magnetic Resonance Spectroscopy
- Higher demands on RF power, due to the higher resonance frequency, leads to increased specific absorption rate (SAR).
- Susceptibility effects increase with field strength, leading to broadening of peaks in spectra. Thus, MRS at higher field makes higher demands on shimming, (i.e., the optimization of the local field homogeneity).
- The increased spectral dispersion at higher fields also requires correspondingly increased bandwidths of radio frequency pulses. This can be accomplished by shortening the pulse length while increasing the amplitude of the transmitted radio frequency’s magnetic field component. This in turn increases SAR and may lead to exceeding the maximum voltage which can be applied to the RF transmit coil.
- The nuclear resonant frequency increases linearly with static field strength, shortening the wavelength of applied RF pulses. This in turn gives rise to a highly inhomogeneous RF excitation.
2. Results and Discussion
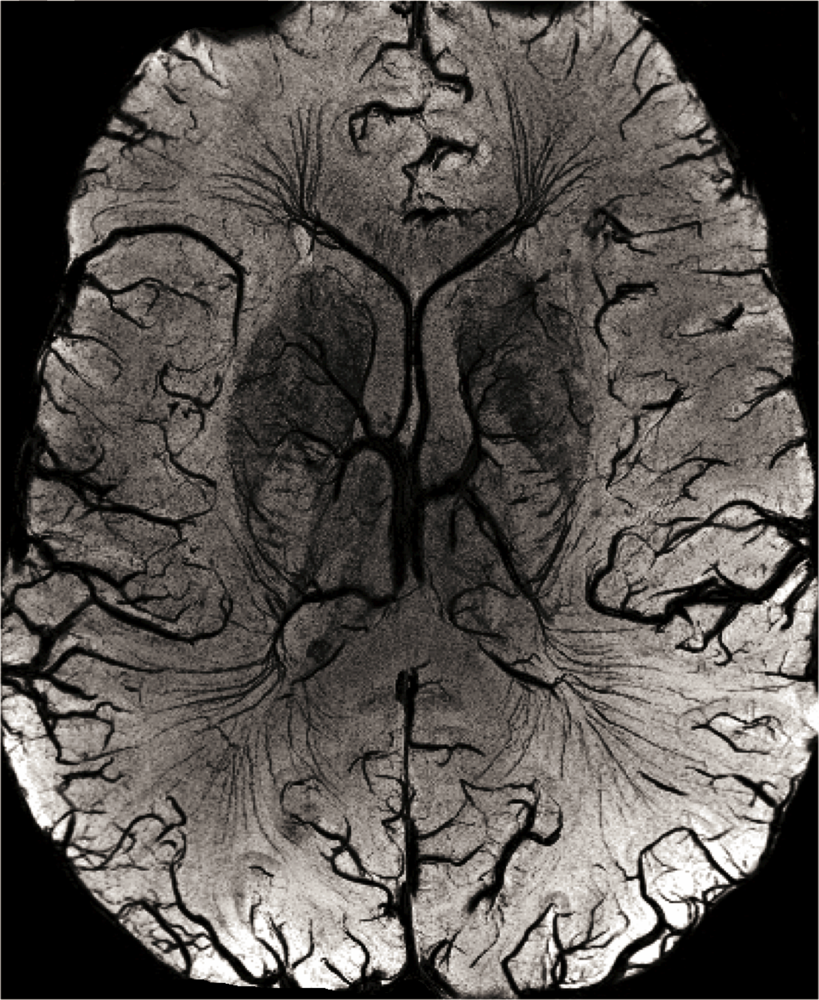
2.1. High-Resolution Structural MRI
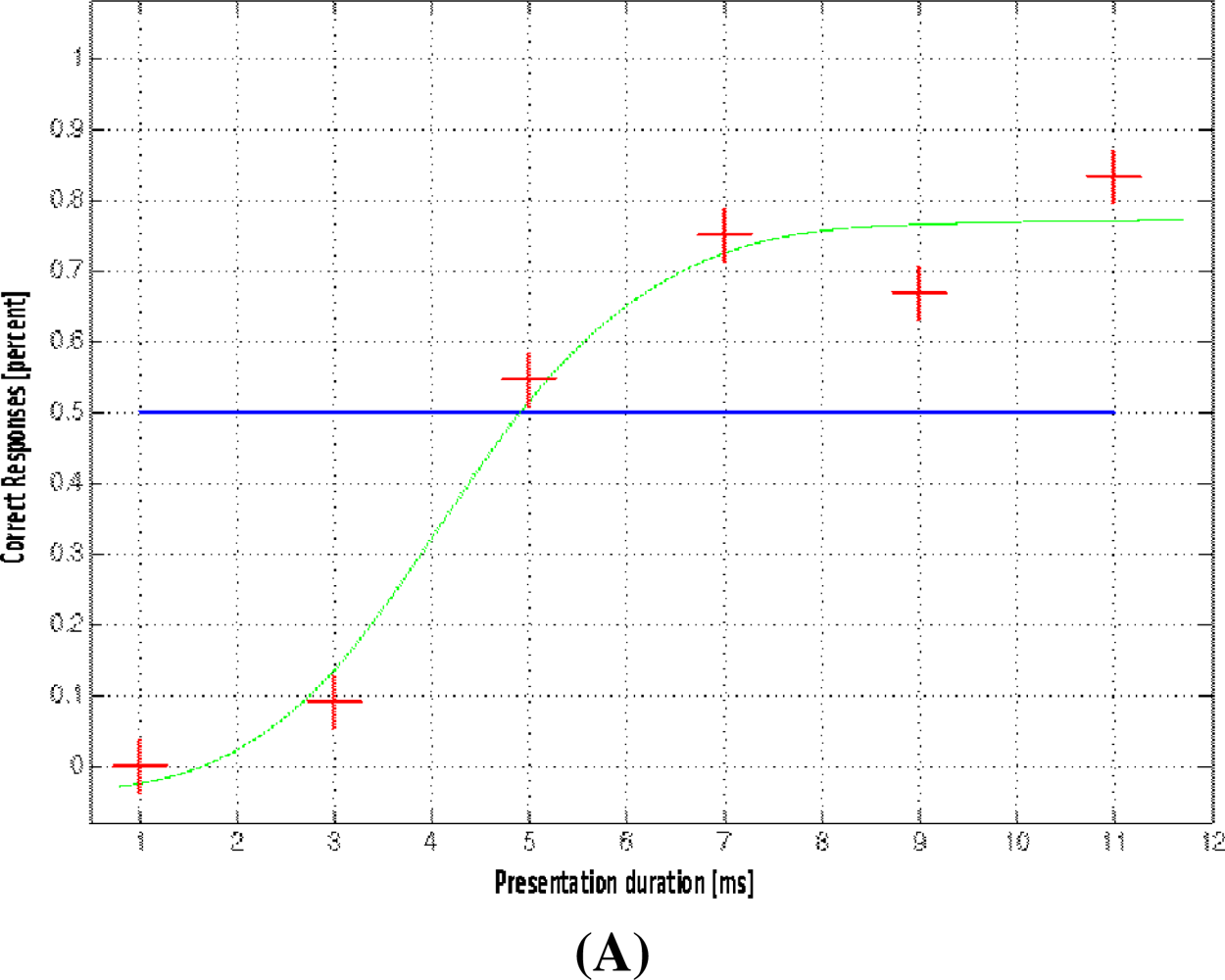
2.2. Functional MRI
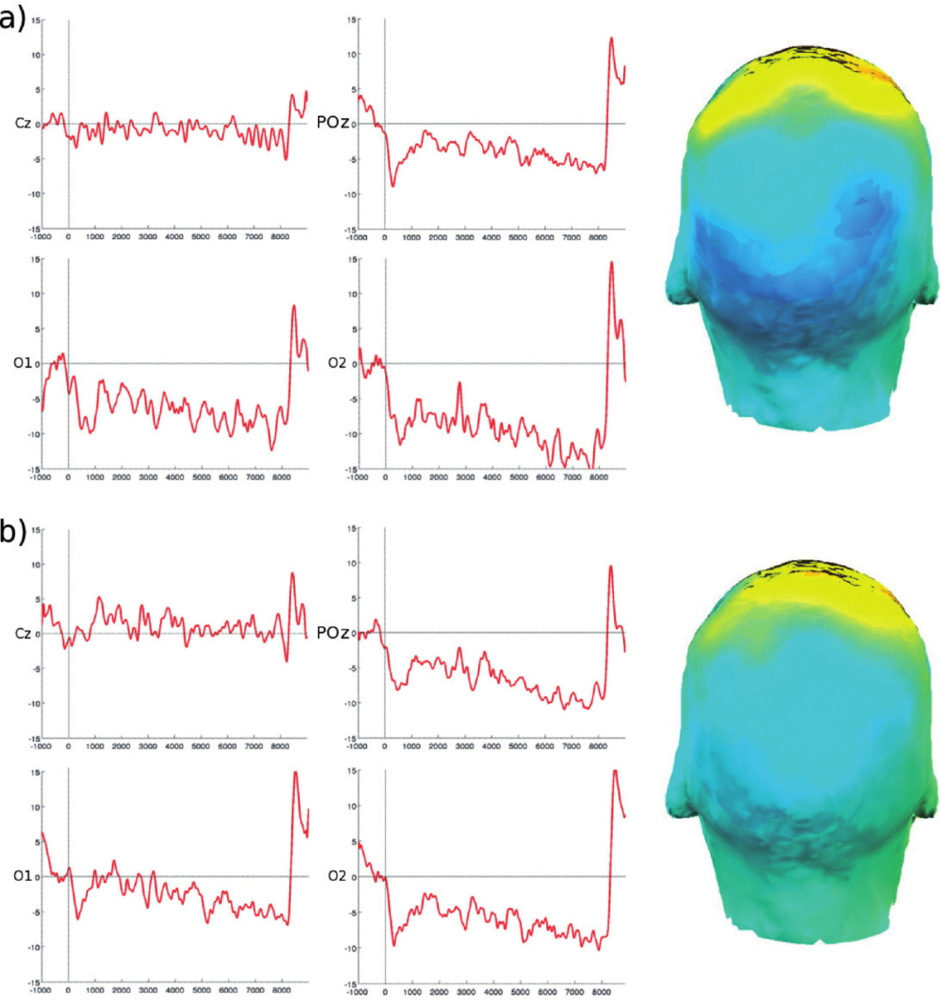
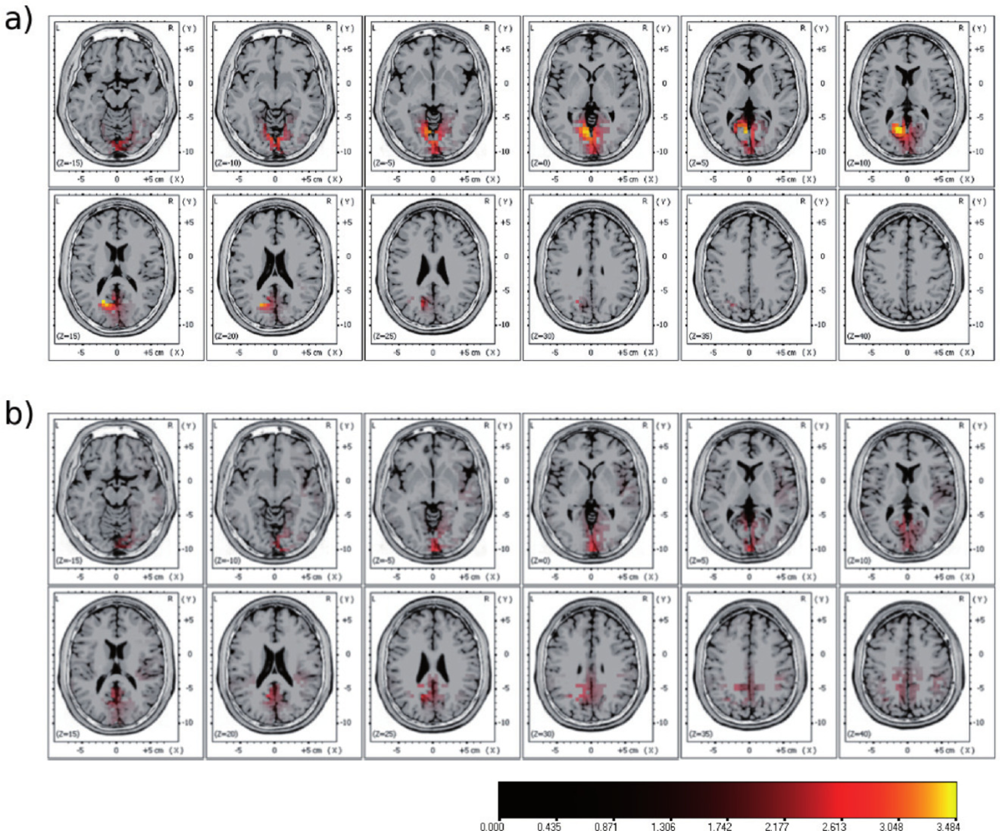
2.3. Multi-Modal Integration of MRI, fMRI, and Slow Cortical Potentials (SCP, DC-EEG)
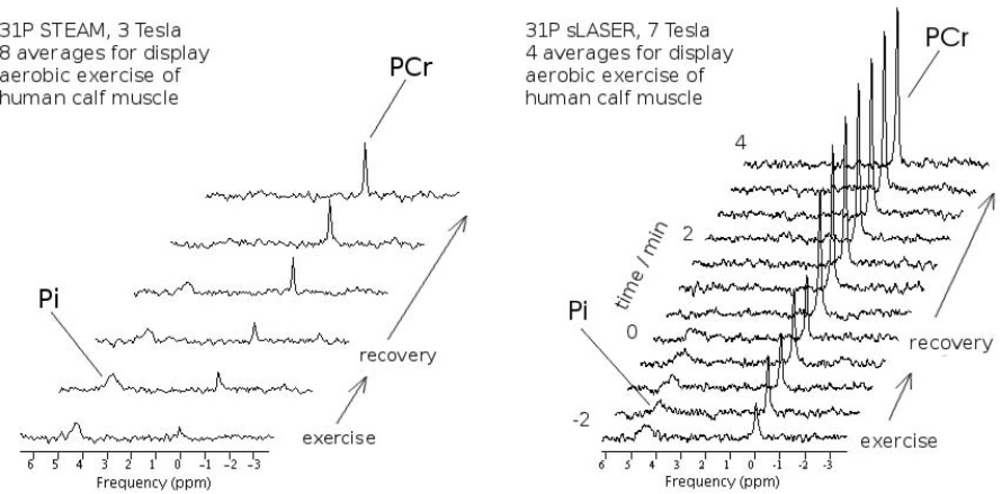
2.4. Magnetic Resonance Spectroscopy
3. Experimental Section
Safety considerations:
Technical Details on MRI Protocols:
Structural brain imaging:
Functional brain imaging:
4. Conclusions
Acknowledgments
References
- Moser, E. Ultra-high-field Magnetic Resonance: why and when? World J. Radiol 2010, 2, 37–40. [Google Scholar]
- Ugurbil, K.; Adriany, G.; Andersen, P.; Chen, W.; Garwood, M.; Gruetter, R.; Henry, P.G.; Kim, S.G.; Lieu, H.; Tkac, I.; Vaughan, T.; van De Moortele, P.F.; Yacoub, E.; Zhu, X.H. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging 2003, 21, 1263–1281. [Google Scholar]
- De Graaf, RA. In Vivo NMR Spectroscopy: Principles and Techniques; Wiley: Chichester, UK; New York, NY, USA, 1998. [Google Scholar]
- Haacke, E.M. Magnetic resonance imaging: physical principles and sequence design; Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Filippi, M. fMRI Techniques and Protocols; Springer: New York, NY, USA, 2009. [Google Scholar]
- He, J.; Farias, S.; Martinez, O.; Reed, B.; Mungas, D.; Decarli, C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch. Neurol 2009, 66, 1393–1399. [Google Scholar]
- Knoops, A.J.; van der Graaf, Y.; Appelman, A.P.; Mali, W.P.; Geerlings, M.I. Total cerebral blood flow and hippocampal volume in patients with arterial disease. The SMART-MR study. J Cereb. Blood Flow Metab 2009, 29, 1727–1733. [Google Scholar]
- de Lange, E.E.; Mugler, J.P.; Bertolina, J.A.; Gay, S.B.; Janus, C.L.; Brookeman, J.R. Magnetization prepared rapid gradient-echo (MP-RAGE) MR imaging of the liver: comparison with spin-echo imaging. Magn. Reson. Imaging 1991, 9, 469–476. [Google Scholar]
- Hennig, J.; Nauerth, A.; Friedburg, H. RARE imaging: a fast imaging method for clinical MR. Magn. Reson. Med 1986, 3, 823–833. [Google Scholar]
- Weigel, M.; Hennig, J. Contrast behavior and relaxation effects of conventional and hyperecho-turbo spin echo sequences at 1.5 and 3 T. Magn. Reson. Med 2006, 55, 826–835. [Google Scholar]
- Haacke, E.M.; Xu, Y.; Cheng, Y.C.; Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med 2004, 52, 612–618. [Google Scholar]
- Cho, Z.H.; Min, H.K.; Oh, S.H.; Han, J.Y.; Park, C.W.; Chi, J.G.; Kim, Y.B.; Paek, S.H.; Lozano, A.M.; Lee, K.H. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J. N eurosurg 2010. [Google Scholar] [CrossRef]
- Theysohn, J.M.; Kraff, O.; Maderwald, S.; Schlamann, M.U.; de Greiff, A.; Forsting, M.; Ladd, S.C.; Ladd, M.E.; Gizewski, E.R. The human hippocampus at 7 T—in vivo MRI. Hippocampus 2009, 19, 1–7. [Google Scholar]
- Calvini, P.; Chincarini, A.; Gemme, G.; Penco, M.A.; Squarcia, S.; Nobili, F.; Rodriguez, G.; Bellotti, R.; Catanzariti, E.; Cerello, P.; De Mitri, I.; Fantacci, M.E. Automatic analysis of medial temporal lobe atrophy from structural MRIs for the early assessment of Alzheimer disease. Med. Phys 2009, 36, 3737–3747. [Google Scholar]
- Nakada, T.; Matsuzawa, H.; Igarashi, H.; Fujii, Y.; Kwee, I.L. In vivo visualization of senile-plaque-like pathology in Alzheimer’s disease patients by MR microscopy on a 7T system. J Neuroimaging 2008, 18, 125–129. [Google Scholar]
- Reichenbach, J.R.; Barth, M.; Haacke, E.M.; Klarhofer, M.; Kaiser, W.A.; Moser, E. High-resolution MR venography at 3.0 Tesla. J. Comput. Assist. Tomogr 2000, 24, 949–957. [Google Scholar]
- Lee, B.C.; Vo, K.D.; Kido, D.K.; Mukherjee, P.; Reichenbach, J.; Lin, W.; Yoon, M.S.; Haacke, M. MR high-resolution blood oxygenation level-dependent venography of occult (low-flow) vascular lesions. AJNR Am. J. Neuroradiol 1999, 20, 1239–1242. [Google Scholar]
- Essig, M.; Reichenbach, J.R.; Schad, L.R.; Schoenberg, S.O.; Debus, J.; Kaiser, W.A. High-resolution MR venography of cerebral arteriovenous malformations. Magn. Reson. Imaging 1999, 17, 1417–1425. [Google Scholar]
- Tan, I.L.; van Schijndel, R.A.; Pouwels, P.J.; van Walderveen, M.A.; Reichenbach, J.R.; Manoliu, R.A.; Barkhof, F. MR venography of multiple sclerosis. AJNR Am. J. Neuroradiol 2000, 21, 1039–1042. [Google Scholar]
- Barth, M.; Nobauer-Huhmann, I.M.; Reichenbach, J.R.; Mlynarik, V.; Schoggl, A.; Matula, C.; Trattnig, S. High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla. Invest. Radiol 2003, 38, 409–414. [Google Scholar]
- Schad, L.R. Improved target volume characterization in stereotactic treatment planning of brain lesions by using high-resolution BOLD MR-venography. NMR Biomed 2001, 14, 478–483. [Google Scholar]
- Haacke, E.M.; Cheng, N.Y.; House, M.J.; Liu, Q.; Neelavalli, J.; Ogg, R.J.; Khan, A.; Ayaz, M.; Kirsch, W.; Obenaus, A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 2005, 23, 1–25. [Google Scholar]
- Shmueli, K.; de Zwart, J.A.; van Gelderen, P.; Li, T.Q.; Dodd, S.J.; Duyn, J.H. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn. Reson. Med 2009, 62, 1510–1522. [Google Scholar]
- Grabner, G.; Haubenberger, D.; Rath, J.; Beisteiner, R.; Auff, E.; Trattnig, S.; Barth, M. A Population-Specific Symmetric Phase Model to Automatically Analyze Susceptibility-Weighted Imaging (SWI) Phase Shifts and Phase Symmetry in the Human Brain. J. Magn. Reson. Imaging 2009, 31, 215–220. [Google Scholar]
- Eckstein, F.; Charles, H.C.; Buck, R.J.; Kraus, V.B.; Remmers, A.E.; Hudelmaier, M.; Wirth, W.; Evelhoch, J.L. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum 2005, 52, 3132–3136. [Google Scholar]
- Chang, G.; Regatte, R.R.; Schweitzer, M.E. Olympic fencers: adaptations in cortical and trabecular bone determined by quantitative computed tomography. Osteoporos. Int 2009, 20, 779–785. [Google Scholar]
- Goldstein, S.A.; Goulet, R.; McCubbrey, D. Measurement and significance of three-dimensional architecture to the mechanical integrity of trabecular bone. Calcif. Tissue Int 1993, 53 Suppl 1. [Google Scholar]
- Kleerekoper, M.; Villanueva, A.R.; Stanciu, J.; Rao, D.S.; Parfitt, A.M. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif. Tissue Int 1985, 37, 594–597. [Google Scholar]
- Borah, B.; Dufresne, T.E.; Chmielewski, P.A.; Gross, G.J.; Prenger, M.C.; Phipps, R.J. Risedronate preserves trabecular architecture and increases bone strength in vertebra of ovariectomized minipigs as measured by three-dimensional microcomputed tomography. J. Bone Miner. Res 2002, 17, 1139–1147. [Google Scholar]
- Ding, M.; Odgaard, A.; Linde, F.; Hvid, I. Age-related variations in the microstructure of human tibial cancellous bone. J. Orthop. Res 2002, 20, 615–621. [Google Scholar]
- Langenberger, H.; Shimizu, Y.; Windischberger, C.; Grampp, S.; Berg, A.; Ferlitsch, K.; Moser, E. Bone homogeneity factor: an advanced tool for the assessment of osteoporotic bone structure in high-resolution magnetic resonance images. Invest. Radiol 2003, 38, 467–472. [Google Scholar]
- Krug, R.; Carballido-Gamio, J.; Banerjee, S.; Burghardt, A.J.; Link, T.M.; Majumdar, S. In vivo ultra-high-field magnetic resonance imaging of trabecular bone microarchitecture at 7 T. J. Magn. Reson. Imaging 2008, 27, 854–859. [Google Scholar]
- Stahl, R.; Krug, R.; Kelley, D.A.; Zuo, J.; Ma, C.B.; Majumdar, S.; Link, T.M. Assessment of cartilage-dedicated sequences at ultra-high-field MRI: comparison of imaging performance and diagnostic confidence between 3.0 and 7.0 T with respect to osteoarthritis-induced changes at the knee joint. Skeletal. Radiol 2009, 38, 771–783. [Google Scholar]
- Kraff, O.; Theysohn, J.M.; Maderwald, S.; Saylor, C.; Ladd, S.C.; Ladd, M.E.; Barkhausen, J. MRI of the knee at 7.0 Tesla. Rofo 2007, 179, 1231–1235. [Google Scholar]
- Bauer, J.S.; Monetti, R.; Krug, R.; Matsuura, M.; Mueller, D.; Eckstein, F.; Rummeny, E.J.; Lochmueller, E.M.; Raeth, C.W.; Link, T.M. Advances of 3T MR imaging in visualizing trabecular bone structure of the calcaneus are partially SNR-independent: analysis using simulated noise in relation to micro-CT, 1.5T MRI, and biomechanical strength. J. Magn. Reson. Imaging 2009, 29, 132–140. [Google Scholar]
- Bauer, J.S.; Banerjee, S.; Henning, T.D.; Krug, R.; Majumdar, S.; Link, T.M. Fast high-spatial-resolution MRI of the ankle with parallel imaging using GRAPPA at 3 T. AJR Am. J. Roentgenol 2007, 189, 240–245. [Google Scholar]
- Barr, C.; Bauer, J.S.; Malfair, D.; Ma, B.; Henning, T.D.; Steinbach, L.; Link, T.M. MR imaging of the ankle at 3 Tesla and 1.5 Tesla: protocol optimization and application to cartilage, ligament and tendon pathology in cadaver specimens. Eur. Radiol 2007, 17, 1518–1528. [Google Scholar]
- Banerjee, S.; Choudhury, S.; Han, E.T.; Brau, A.C.; Morze, C.V.; Vigneron, D.B.; Majumdar, S. Autocalibrating parallel imaging of in vivo trabecular bone microarchitecture at 3 Tesla. Magn. Reson. Med 2006, 56, 1075–1084. [Google Scholar]
- Banerjee, S.; Krug, R.; Carballido-Gamio, J.; Kelley, D.A.; Xu, D.; Vigneron, D.B.; Majumdar, S. Rapid in vivo musculoskeletal MR with parallel imaging at 7T. Magn. Reson. Med 2008, 59, 655–660. [Google Scholar]
- Anderson, M.L.; Skinner, J.A.; Felmlee, J.P.; Berger, R.A.; Amrami, K.K. Diagnostic comparison of 1.5 Tesla and 3.0 Tesla preoperative MRI of the wrist in patients with ulnar-sided wrist pain. J. Hand Surg. Am 2008, 33, 1153–1159. [Google Scholar]
- Lenk, S.; Ludescher, B.; Martirosan, P.; Schick, F.; Claussen, C.D.; Schlemmer, H.P. 3.0 T high-resolution MR imaging of carpal ligaments and TFCC. Rofo 2004, 176, 664–667. [Google Scholar]
- Farooki, S.; Ashman, C.J.; Yu, J.S.; Abduljalil, A.; Chakeres, D. In vivo high-resolution MR imaging of the carpal tunnel at 8.0 tesla. Skeletal. Radiol 2002, 31, 445–450. [Google Scholar]
- Behr, B.; Stadler, J.; Michaely, H.J.; Damert, H.G.; Schneider, W. MR imaging of the human hand and wrist at 7 T. Skeletal. Radiol. 2009, 38, 911–917. [Google Scholar]
- Friedrich, K.M.; Chang, G.; Vieira, R.L.; Wang, L.; Wiggins, G.C.; Schweitzer, M.E.; Regatte, R.R. In vivo 7.0-tesla magnetic resonance imaging of the wrist and hand: technical aspects and applications. Semin. Musculoskelet. Radiol. 2009, 13, 74–84. [Google Scholar]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD signal. Annu. Rev. Physiol 2004, 66, 735–769. [Google Scholar]
- Merboldt, K.D.; Fransson, P.; Bruhn, H.; Frahm, J. Functional MRI of the human amygdala? Neuroimage 2001, 14, 253–257. [Google Scholar]
- Robinson, S.; Windischberger, C.; Rauscher, A.; Moser, E. Optimized 3 T EPI of the amygdalae. Neuroimage 2004, 22, 203–210. [Google Scholar]
- Robinson, S.D.; Pripfl, J.; Bauer, H.; Moser, E. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the Default Mode. MAGMA 2008, 21, 279–290. [Google Scholar]
- Windischberger, C.; Cunnington, R.; Lamm, C.; Lanzenberger, R.; Langenberger, H.; Deecke, L.; Bauer, H.; Moser, E. Time-resolved analysis of fMRI signal changes using Brain Activation Movies. J. Neurosci. Methods 2008, 169, 222–230. [Google Scholar]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med 1995, 34, 537–541. [Google Scholar]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar]
- Robinson, S.; Basso, G.; Soldati, N.; Sailer, U.; Jovicich, J.; Bruzzone, L.; Kryspin-Exner, I.; Bauer, H.; Moser, E. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci 2009, 10, 137. [Google Scholar]
- Greicius, M.D.; Boyett-Anderson, J.M.; Menon, V.; Reiss, A.L. Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome. Neuroreport 2004, 15, 1579–1583. [Google Scholar]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 2009, 47, 1408–1416. [Google Scholar]
- Kouider, S.; Dehaene, S. Levels of processing during non-conscious perception: a critical review of visual masking. Philos. Trans. R Soc. Lond B Biol. Sci 2007, 362, 857–875. [Google Scholar]
- Paus, T. Functional anatomy of arousal and attention systems in the human brain. Prog. Brain Res 2000, 126, 65–77. [Google Scholar]
- Dehaene, S.; Changeux, J.P.; Naccache, L.; Sackur, J.; Sergent, C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn. Sci 2006, 10, 204–211. [Google Scholar]
- Zeki, S. The disunity of consciousness. Trends Cogn. Sci 2003, 7, 214–218. [Google Scholar]
- Hamalainen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput 1994, 32, 35–42. [Google Scholar]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp. Clin. Pharmacol 2002, 24 Suppl D, 5–12. [Google Scholar]
- Ives, J.R.; Warach, S.; Schmitt, F.; Edelman, R.R.; Schomer, D.L. Monitoring the patient’s EEG during echo planar MRI. Electroencephalogr. Clin. Neurophysiol 1993, 87, 417–420. [Google Scholar]
- Rosenkranz, K.; Lemieux, L. Present and future of simultaneous EEG-fMRI. MAGMA 2010. [Google Scholar] [CrossRef]
- Debener, S.; Mullinger, K.J.; Niazy, R.K.; Bowtell, R.W. Properties of the ballistocardiogram artefact as revealed by EEG recordings at 1.5, 3 and 7 T static magnetic field strength. Int. J. Psychophysiol 2008, 67, 189–199. [Google Scholar]
- Yan, W.X.; Mullinger, K.J.; Brookes, M.J.; Bowtell, R. Understanding gradient artefacts in simultaneous EEG/fMRI. Neuroimage 2009, 46, 459–471. [Google Scholar]
- Ritter, P.; Villringer, A. Simultaneous EEG-fMRI. Neurosci. Biobehav. Rev 2006, 30, 823–838. [Google Scholar]
- Fields, R.D.; Stevens, B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 2000, 23, 625–633. [Google Scholar]
- Fields, R.D.; Stevens-Graham, B. New insights into neuron-glia communication. Science 2002, 298, 556–562. [Google Scholar]
- Logothetis, N.K. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R Soc. Lond B Biol. Sci 2002, 357, 1003–1037. [Google Scholar]
- Lamm, C.; Windischberger, C.; Leodolter, U.; Moser, E.; Bauer, H. Evidence for premotor cortex activity during dynamic visuospatial imagery from single-trial functional magnetic resonance imaging and event-related slow cortical potentials. Neuroimage 2001, 14, 268–283. [Google Scholar]
- Khader, P.; Schicke, T.; Roder, B.; Rosler, F. On the relationship between slow cortical potentials and BOLD signal changes in humans. Int. J. Psychophysiol 2008, 67, 252–261. [Google Scholar]
- He, B.J.; Snyder, A.Z.; Zempel, J.M.; Smyth, M.D.; Raichle, M.E. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. USA 2008, 105, 16039–16044. [Google Scholar]
- Mankin, H.J. Biochemical and metabolic aspects of osteoarthritis. Orthop. Clin. North. Am 1971, 2, 19–31. [Google Scholar]
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: structure and potential functions. Microsc. Res. Tech 1994, 28, 385–397. [Google Scholar]
- Lohmander, L.S. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J. Anat 1994, 184, 477–492. [Google Scholar]
- Grushko, G.; Schneiderman, R.; Maroudas, A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect. Tissue Res 1989, 19, 149–176. [Google Scholar]
- Maroudas, A.; Muir, H.; Wingham, J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim. Biophys. Acta 1969, 177, 492–500. [Google Scholar]
- Borthakur, A.; Shapiro, E.M.; Beers, J.; Kudchodkar, S.; Kneeland, J.B.; Reddy, R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage 2000, 8, 288–293. [Google Scholar]
- Shapiro, E.M.; Borthakur, A.; Gougoutas, A.; Reddy, R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn. Reson. Med 2002, 47, 284–291. [Google Scholar]
- Borthakur, A.; Shapiro, E.M.; Akella, S.V.; Gougoutas, A.; Kneeland, J.B.; Reddy, R. Quantifying sodium in the human wrist in vivo by using MR imaging. Radiology 2002, 224, 598–602. [Google Scholar]
- Lesperance, L.M.; Gray, M.L.; Burstein, D. Determination of fixed charge density in cartilage using nuclear magnetic resonance. J. Orthop. Res 1992, 10, 1–13. [Google Scholar]
- Shapiro, E.M.; Borthakur, A.; Dandora, R.; Kriss, A.; Leigh, J.S.; Reddy, R. Sodium visibility and quantitation in intact bovine articular cartilage using high field 23Na MRI and MRS. J. Magn. Reson 2000, 142, 24–31. [Google Scholar]
- Burstein, D.; Velyvis, J.; Scott, K.T.; Stock, K.W.; Kim, Y.J.; Jaramillo, D.; Boutin, R.D.; Gray, M.L. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI: (dGEMRIC) for clinical evaluation of articular cartilage. Magn. Reson. Med 2001, 45, 36–41. [Google Scholar]
- Kemp, G.J.; Meyerspeer, M.; Moser, E. Absolute quantitation of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed 2007, 20, 555–565. [Google Scholar]
- Meyerspeer, M.; Kemp, G.J.; Mlynarik, V.; Krssak, M.; Szendroedi, J.; Nowotny, P.; Roden, M.; Moser, E. Direct noninvasive quantification of lactate and high energy phosphates simultaneously in exercising human skeletal muscle by localized magnetic resonance spectroscopy. Magn. Reson. Med 2007, 57, 654–660. [Google Scholar]
- Schmid, A.I.; Chmelik, M.; Szendroedi, J.; Krssak, M.; Brehm, A.; Moser, E.; Roden, M. Quantitative ATP synthesis in human liver measured by localized 31P spectroscopy using the magnetization transfer experiment. NMR Biomed 2008, 21, 437–443. [Google Scholar]
- Gruber, S.; Pinker, K.; Riederer, F.; Chmelík, M.; Stadlbauer, A.; Bittsansky, M.; Mlynárik, V.; Frey, R.; Serles, W.; Bodamer, O.; Moser, E. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur. J. Radiol 2008, 68, 320–327. [Google Scholar]
- Szendroedi, J.; Schmid, A.I.; Meyerspeer, M.; Cervin, C.; Kacerovsky, M.; Smekal, G.; Gräser-Lang, S.; Groop, L.; Roden, M. Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care 2009, 32, 677–679. [Google Scholar]
- Krssak, M.; Krebs, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Multinuclear 1H, 13C and 31P NMRS study on mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Proc. Intl. Soc. Mag. Reson. Med. (ISMRM), 10th Annual Meeting 2002, 10, 170. [Google Scholar]
- Stadlbauer, A.; Gruber, S.; Nimsky, C.; Fahlbusch, R.; Hammen, T.; Buslei, R.; Tomandl, B.; Moser, E.; Ganslandt, O. Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology 2006, 238, 958–969. [Google Scholar]
- Krssak, M.; Petersen, K.F.; Bergeron, R.; Price, T.; Laurent, D.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J. Clin. Endocrinol. Metab 2000, 85, 748–754. [Google Scholar]
- Schick, F.; Eismann, B.; Jung, W.I.; Bongers, H.; Bunse, M.; Lutz, O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn. Reson. Med 1993, 29, 158–167. [Google Scholar]
- Mangia, S.; Tkac, I.; Gruetter, R.; van De Moortele, P.F.; Giove, F.; Maraviglia, B.; Ugurbil, K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn. Reson. Imaging 2006, 24, 343–348. [Google Scholar]
- Tkac, I.; Andersen, P.; Adriany, G.; Merkle, H.; Ugurbil, K.; Gruetter, R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn. Reson. Med 2001, 46, 451–456. [Google Scholar]
- Mangia, S.; Giove, F.; Tkac, I.; Logothetis, N.K.; Henry, P.G.; Olman, C.A.; Maraviglia, B.; Di Salle, F.; Ugurbil, K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J. Cereb. Blood Flow Metab 2009, 29, 441–463. [Google Scholar]
- Tkac, I.; Oz, G.; Adriany, G.; Ugurbil, K.; Gruetter, R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn. Reson. Med 2009, 62, 868–879. [Google Scholar]
- Scheenen, T.W.; Heerschap, A.; Klomp, D.W. Towards 1H-MRSI of the human brain at 7 T with slice-selective adiabatic refocusing pulses. MAGMA 2008, 21, 95–101. [Google Scholar]
- Gruber, S.; Stadlbauer, A.; Mlynarík, V.; Gatterbauer, B.; Roessler, K.; Moser, E. Proton magnetic resonance spectroscopic imaging in brain tumor diagnosis. Neurosurg. Clin. N Am 2005, 16, 101–114. [Google Scholar]
- Bogner, W.; Gruber, S.; Doelken, M.; Stadlbauer, A.; Ganslandt, O.; Boettcher, U.; Trattnig, S.; Doerfler, A.; Stefan, H.; Hammen, T. In vivo quantification of intracerebral GABA by single-voxel 1H-MRS-How reproducible are the results. Eur. J. Radiol 2009, 73, 526–531. [Google Scholar]
- Kemp, G.J.; Radda, G.K. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn. Reson. Q 1994, 10, 43–63. [Google Scholar]
- Du, F.; Zhu, X.H.; Qiao, H.; Zhang, X.; Chen, W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn. Reson. Med 2007, 57, 103–114. [Google Scholar]
- Meyer, R.A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am. J. Physiol 1988, 254, C548–553. [Google Scholar]
- Meyerspeer, M.; Scheenen, T.; Moser, E. Dynamic 31P MRS of exercising human muscle in a 7 T whole body system, with STEAM and semi-LASER localisation. Proc. Intl. Soc. Mag. Reson. Med (ISMRM), 17th Annual Meeting; 2009. # 2590.. [Google Scholar]
- Chance, B.; Leigh, J.S.; Kent, J.; McCully, K.; Nioka, S.; Clark, B.J.; Maris, J.M.; Graham, T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc. Natl. Acad. Sci. USA 1986, 83, 9458–9462. [Google Scholar]
- Kemp, G.J. Studying metabolic regulation in human muscle. Biochem. Soc. Trans 2000, 28, 100–103. [Google Scholar]
- Meyerspeer, M.; Krssak, M.; Kemp, G.J.; Roden, M.; Moser, E. Dynamic interleaved 1H/31P STEAM MRS at 3 Tesla using a pneumatic force-controlled plantar flexion exercise rig. Magn. Reson. Mater. Phy 2005, 18, 257–262. [Google Scholar]
- Brillault-Salvat, C.; Giacomini, E.; Jouvensal, L.; Wary, C.; Bloch, G.; Carlier, P.G. Simultaneous determination of muscle perfusion and oxygenation by interleaved NMR plethysmography and deoxymyoglobin spectroscopy. NMR Biomed 1997, 10, 315–323. [Google Scholar]
- Gruetter, R.; Weisdorf, S.A.; Rajanayagan, V.; Terpstra, M.; Merkle, H.; Truwit, C.L.; Garwood, M.; Nyberg, S.L.; Ugurbil, K. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J. Magn. Reson 1998, 135, 260–264. [Google Scholar]
- Meyerspeer, M.; Krssak, M.; Moser, E. Relaxation Times of 31P-Metabolites in Human Calf Muscle at 3 Tesla. Magn. Reson. Med 2003, 49, 620–625. [Google Scholar]
- Bogner, W.; Chmelik, M.; Schmid, A.I.; Moser, E.; Trattnig, S.; Gruber, S. Assessment of 31P relaxation times in the human calf muscle: a comparison between 3 T and 7 T in vivo. Magn. Reson. Med 2009, 62, 574–582. [Google Scholar]
- Trattnig, S.; Welsch, G.H.; Juras, V.; Szomolanyi, P.; Mayerhoefer, M.; Stelzeneder, D.; Mamisch, T.C.; Bieri, O.; Scheffler, K.; Zbyn, S. 23Na (Sodium) Imaging at 7 T—quantification of glycosaminoglycan content in repair tissue of patients after matrix-associated autologous chondrocyte transplantation in the knee joint: preliminary results. Radiology 2010. accepted.. [Google Scholar]
- Fischmeister, F.P.; Leodolter, U.; Windischberger, C.; Kasess, C.H.; Schopf, V.; Moser, E.; Bauer, H. Multiple serial picture presentation with millisecond resolution using a three-way LC-shutter-tachistoscope. J. Neurosci. Methods 2010, 187, 235–242. [Google Scholar]
- Korunka, C.; Gruber, G.; Leodolter, M.; Bauer, H. Technical Requirements for Long-Time DC-EEG Recordings. Int. J. Psychophysiol 1989, 7, 269–270. [Google Scholar]
- Schlegelmilch, F.; Schellhorn, K.; Markert, S. A model for on-line correction of DC-EEG measurements during fMRI. Neuroimage 2004. OHBM Abstracts.. [Google Scholar]
- Bauer, H.; Lauber, W. Operant conditioning of brain steady potential shifts in man. Biofeedback Self-Regul 1979, 4, 145–154. [Google Scholar]
- Meyerspeer, M.; Unger, E.; Mandl, T.; Scheenen, T.; Kemp, G.J.; Moser, E. Localised versus unlocalised dynamic 31P MRS acquisition in exercising human muscle at 7 T. Proc. Intl. Soc. Mag. Reson. Med. (ISMRM), 18th Annual Meeting; 2010. # 4102.. [Google Scholar]
- Moser, E.; Stadlbauer, A.; Windischberger, C.; Quick, H.H.; Ladd, M.E. Magnetic resonance imaging methodology. Eur. J. Nucl. Med. Mol. Imaging 2009, 36 Suppl 1, S30–41. [Google Scholar]
- Bottomley, P.A.; Andrew, E.R. RF magnetic field penetration, phase shift and power dissipation in biological tissue: implications for NMR imaging. Phys. Med. Biol 1978, 23, 630–643. [Google Scholar]
- Bomsdorf, H.; Helzel, T.; Kunz, D.; Röschmann, P.; Tschendel, O.; Wieland, J. Spectroscopy and imaging with a 4 Tesla whole-body MR system. NMR Biomed 1988, 1, 151–158. [Google Scholar]
- van de Moortele, P.-F.; Akgun, C.; Adriany, G.; Moeller, S.; Ritter, J.; Collins, C.M.; Smith, M.B.; Vaughan, J.T.; Ugurbil, K. B1 destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn. Reson. Med 2005, 54, 1503–1518. [Google Scholar]
- Collins, C.M.; Liu, W.; Schreiber, W.; Yang, Q.X.; Smith, M.B. Central brightening due to constructive interference with, without, and despite dielectric resonance. J. Magn. Reson. Imaging 2005, 21, 192–196. [Google Scholar]
- Vaughan, J.T.; Adriany, G.; Snyder, C.J.; Tian, J.; Thiel, T.; Bolinger, L.; Liu, H.; DelaBarre, L.; Ugurbil, K. Efficient high-frequency body coil for high-field MRI. Magn. Reson. Med 2004, 52, 851–859. [Google Scholar]
- Zelinski, A.C.; Wald, L.L.; Setsompop, K.; Goyal, V.K.; Adalsteinsson, E. Sparsity-enforced slice-selective MRI RF excitation pulse design. IEEE Trans. Med. Imaging 2008, 27, 1213–1229. [Google Scholar]
- Vaughan, J.T.; Snyder, C.J.; DelaBarre, L.J.; Bolan, P.J.; Tian, J.; Bolinger, L.; Adriany, G.; Andersen, P.; Strupp, J.; Ugurbil, K. Whole-body imaging at 7 T: preliminary results. Magn. Reson. Med 2009, 61, 244–248. [Google Scholar]
- Katscher, U.; Börnert, P.; Leussler, C.; van den Brink, J. Transmit SENSE. Magn. Reson. Med 2003, 49, 144–150. [Google Scholar]
- Zhu, Y. Parallel excitation with an array of transmit coils. Magn. Reson. Med 2004, 51, 775–784. [Google Scholar]
- Pauly, J.; Nishimura, D.; Macovski, A. A k-space analysis of small-tip angle excitation. J. Magn. Reson 1989, 81, 43–56. [Google Scholar]
- Wang, Z.; Lin, J.C.; Mao, W.; Liu, W.; Smith, M.B.; Collins, C.M. SAR and temperature: simulations and comparison to regulatory limits for MRI. J. Magn. Reson. Imaging 2007, 26, 437–441. [Google Scholar]
- Collins, C.M.; Smith, M.B. Calculations of B1 distribution, SNR, and SAR for a surface coil adjacent to an anatomically-accurate human body model. Magn. Reson. Med 2001, 45, 692–699. [Google Scholar]
- Collins, C.M.; Liu, W.; Wang, J.; Gruetter, R.; Vaughan, J.T.; Ugurbil, K.; Smith, M.B. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J. Magn. Reson. Imaging 2004, 19, 650–656. [Google Scholar]
- Chen, C.N.; Sank, V.J.; Cohen, S.M.; Hoult, D.I. The field dependence of NMR imaging. I. Laboratory assessment of signal-to-noise ratio and power deposition. Magn. Reson. Med 1986, 3, 722–729. [Google Scholar]
- Hoult, D.I.; Chen, C.N.; Sank, V.J. The field dependence of NMR imaging. II. Arguments concerning an optimal field strength. Magn. Reson. Med 1986, 3, 730–746. [Google Scholar]








© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moser, E.; Meyerspeer, M.; Fischmeister, F.P.S.; Grabner, G.; Bauer, H.; Trattnig, S. Windows on the Human Body – in Vivo High-Field Magnetic Resonance Research and Applications in Medicine and Psychology. Sensors 2010, 10, 5724-5757. https://doi.org/10.3390/s100605724
Moser E, Meyerspeer M, Fischmeister FPS, Grabner G, Bauer H, Trattnig S. Windows on the Human Body – in Vivo High-Field Magnetic Resonance Research and Applications in Medicine and Psychology. Sensors. 2010; 10(6):5724-5757. https://doi.org/10.3390/s100605724
Chicago/Turabian StyleMoser, Ewald, Martin Meyerspeer, Florian Ph. S. Fischmeister, Günther Grabner, Herbert Bauer, and Siegfried Trattnig. 2010. "Windows on the Human Body – in Vivo High-Field Magnetic Resonance Research and Applications in Medicine and Psychology" Sensors 10, no. 6: 5724-5757. https://doi.org/10.3390/s100605724



