QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Layer Optimization
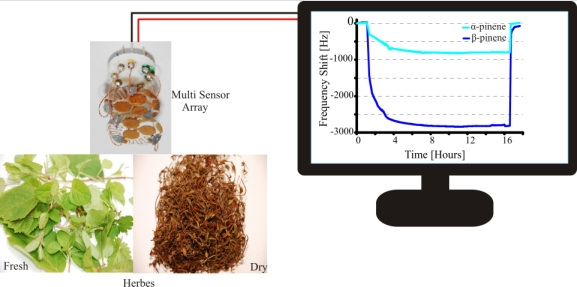
2.2. Selectivity Pattern of the E-nose
2.3. Sensitivity Profile Established from Fresh and Dried Herbs
2.4. Effect on Selectivity of E-nose at Maximum Response from Fresh and Dried Herbs
2.5. Determination of Shelf-Life and Usability of Fresh and Dried Herbs via E-Nose
3. Experimental Section
3.1. Materials and Chemicals
3.2. Measuring Setup
3.3. Head Space Gas Chromatography-Mass Spectrometry (HS, GC-MS)
3.4. Manufacturing of Sensor Array
3.5. Online Measurement Strategy
4. Conclusions
Acknowledgments
References
- Bouvier-Brown, NC; Holzinger, R; Palitzsch, K; Goldstein, AH. Quantifying sesquiterpene and oxygenated terpene emissions from live vegetation using solid-phase microextraction fibers. J. Chromatogr. A 2007, 1161, 113–120. [Google Scholar]
- Wennberg, PO; Hanisco, TF; Jaegle, L; Jacob, DJ; Hintsa, EJ; Lanzendorf, EJ; Anderson, JG; Gao, RS; Keim, ER; Donnelly, SG; Del Negro, LA; Fahey, DW; McKeen, SA; Salawitch, RJ; Webster, CR; May, RD; Herman, RL; Proffitt, MH; Margitan, JJ; Atlas, EL; Schauffler, SM; Flocke, F; McElroy, CT; Bui, TP. Hydrogen radicals, nitrogen radicals, and production of o3 in the upper troposphere. Science 1998, 279, 49–53. [Google Scholar]
- Ka, MH; Choi, EH; Chun, HS; Lee, KG. Antioxidative activity of volatile extracts isolated from angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J. Agric. Food Chem 2005, 53, 4124–4129. [Google Scholar]
- Wayne, RP. Chemistry of Atmospheres, 3rd ed; Oxford University Press: Oxford, UK, 2000; pp. 349–360. [Google Scholar]
- Fick, J; Pommer, L; Andersson, B; Nilsson, C. Ozone removal in the sampling of parts per billion levels of terpenoid compounds: an evaluation of different scrubber materials. Environ. Sci. Technol 2001, 35, 1458–1462. [Google Scholar]
- Cometto-Muniz, JE; Cain, WS; Abraham, MH; Kumarsingh, R. Sensory properties of selected terpenes: thresholds for odor, nasal pungency, nasal localization, and eye irritation. Ann. NY Acad. Sci 1998, 855, 648–651. [Google Scholar]
- Lee, SK; Kim, JH; Sohn, HJ; Yang, JW. Changes in aroma characteristics during the preparation of red ginseng estimated by electronic nose, sensory evaluation and gas chromatography/mass spectrometry. Sens. Actuat. B: Chem 2005, 106, 7–12. [Google Scholar]
- DaruháziA, E; Szarka, S; Héthelyi, E; Simándi, B; Gyurján, I; László, M; Szőke, E; Lemberkovics, E. GC-MS Identification and GC-FID Quantitation of Terpenoids in Ononidis spinosae. Radix. DaruháziA, E.; Szarka, S.; Héthelyi, E.; Simándi, B.; Gyurján, I.; László, M.; Szőke, E.; Lemberkovics, E. GC-MS Identification and GC-FID Quantitation of Terpenoids in Ononidis spinosae. Radix.. Chromatographia Suppl 2008, 68, S71–S76. [Google Scholar]
- Fojtová, J; Lojková, L; Kubáň, V. GC/MS of Terpenes in Walnut-Tree Leaves after Accelerated Solvent Extraction. J. Sep. Sci 2008, 31, 162–168. [Google Scholar]
- Jetti, RR; Yang, AK; Finn, C; Qian, MC. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci 2007, 72, S487–S496. [Google Scholar]
- Allen, MR; Braithwaite, A; Hills, CC. Trace organic compounds in landfill gas at seven UK waste disposal sites. Environ. Sci. Technol 1997, 31, 1054–1061. [Google Scholar]
- Lieberzeit, PA; Rehman, A; Najafi, B; Dickert, FL. Real-life application of a qcm-based e-nose: quantitative characterization of different plant-degradation processes. Anal. Bioanal. Chem 2008, 391, 2897–2903. [Google Scholar]
- Barbria, NE; Mirhissea, J; Ionescub, R; El Baric, N; Correigb, X; Bouchikhia, B; Llobetb, E. An electronic nose system based on a micro-machined gas sensor array to assess the freshness of sardines. Sens. Actuat. B: Chem 2009, 141, 538–543. [Google Scholar]
- Moreno, CL; Kloock, JP; Schoening, MJ; Baldi, A; Ipatov, A; Bratov, A; Jimenez-Jorquera, C. Electronic integrated multisensor tongue applied to grape juice and wine analysis. Analyst 2008, 133, 1440–1448. [Google Scholar]
- Dias, LA; Peres, AM; Vilas, BM; Rocha, MA; Estevinho, L; Machado; Adelio, ASC. An electronic tongue for honey classification. Microchim. Acta 2008, 163, 97–102. [Google Scholar]
- Dickert, FL; Lieberzeit, PA. Imprinted polymers in chemical recognition for mass-sensitive devices. Springer Ser. Chem. Sens. Biosens 2007, 5, 173–210. [Google Scholar]
- Wulff, G. Molecular imprinting in crosslinked materials with the aid of molecular templates - a way towards artificial antibodies. Angew.Chem. Int. Ed 1995, 34, 1812–32. [Google Scholar]
- Dickert, FL; Lieberzeit, PA; Achatz, P; Palfinger, C; Fassnauer, M; Schmid, E; Werther, W; Horner, G. QCM array for on-line-monitoring of composting procedures. Analyst 2004, 129, 432–437. [Google Scholar]
- Kikuchi, M; Tsuru, N; Shiratori, S. Recognition of terpenes using molecular imprinted polymer coated quartz crystal microbalance in air phase. Sci. Technol. Adv. Mater 2006, 7, 156–161. [Google Scholar]
- Percival, CJ; Stanley, S; Galle, M; Braithwaite, A; Newton, MI; McHale, G; Hayes, W. Molecular-imprinted, polymer-coated quartz crystal microbalances for the detection of terpenes. Anal. Chem 2001, 73, 4225–4228. [Google Scholar]
- Loughrin, JH; Kasperbauer, MJ. Aroma content of fresh basil (ocimum basilicum l.) leaves is affected by light reflected from colored mulches. J. Agric. Food Chem 2003, 51, 2272–2276. [Google Scholar]
- Baritaux, O; Richard, H; Touche, J; Derbesy, M. Effects of drying and storage of herbs and spices on the essential oil. Part I. Basil, Ocimum basilicum L. Flavour Fragr. J 1992, 7, 267–71. [Google Scholar]
- Flamini, G; Cioni, PL; Morelli, I; Macchia, M; Ceccarini, L. Main agronomic-productive characteristics of two ecotypes of rosmarinus officinalis l. and chemical composition of their essential oils. J. Agric. Food Chem 2002, 50, 3512–3517. [Google Scholar]
- Valeria, R; Silvia, C; Eliza, O. Capillary gas chromatography—mass spectrometry of volatile compounds of salvia officinalis. J. Chromatogr. A 2004, 1027, 121–126. [Google Scholar]
- Limbo, S; Sinelli, N; Torri, L; Riva, M. Freshness decay and shelf life predictive modeling of european sea bass (dicentrarchus labrax) applying chemical methods and electronic nose. LW Food Sci. Technol 2009, 42, 977–984. [Google Scholar]
- Lieberzeit, PA; Rehman, A; Iqbal, N; Najafi, B; Dickert, FL. QCM Sensor Array For Monitoring Terpene Emissions From Odoriferous Plants. Chem. Mon 2009, 140, 947–952. [Google Scholar]










| Layers | Composition (μL) | Normalized Response (Hz) | ||||
|---|---|---|---|---|---|---|
| Styrene | DVB | Template | 25 ppm Limonene | 50 ppm Eucalyptol | ||
| 1 | 1 | 1.5 | 0 | 0.98 | 1.23 | |
| 2 | 1 | 1.5 | 5 | 3.81 | 4.84 | |
| 3 | 2 | 1.5 | 5 | 3.13 | 4.17 | |
| 4 | 1 | 1.5 | 10 | 6.92 | 6.39 | |
| 5 | 2 | 1.5 | 10 | 4.81 | 3.45 | |
| 6 | 1 | 1.5 | 15 | 5.41 | 6.01 | |
| 7 | 2 | 1.5 | 15 | 4.97 | 5.77 | |
© 2010 by the authors licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers. Sensors 2010, 10, 6361-6376. https://doi.org/10.3390/s100706361
Iqbal N, Mustafa G, Rehman A, Biedermann A, Najafi B, Lieberzeit PA, Dickert FL. QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers. Sensors. 2010; 10(7):6361-6376. https://doi.org/10.3390/s100706361
Chicago/Turabian StyleIqbal, Naseer, Ghulam Mustafa, Abdul Rehman, Alexander Biedermann, Bita Najafi, Peter A. Lieberzeit, and Franz L. Dickert. 2010. "QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers" Sensors 10, no. 7: 6361-6376. https://doi.org/10.3390/s100706361