A Colorimetric Sensor for Qualitative Discrimination and Quantitative Detection of Volatile Amines
Abstract
:1. Introduction
2. Experiments
2.1. CSAs Preparation
2.2. Colorimetric Sensor Construction
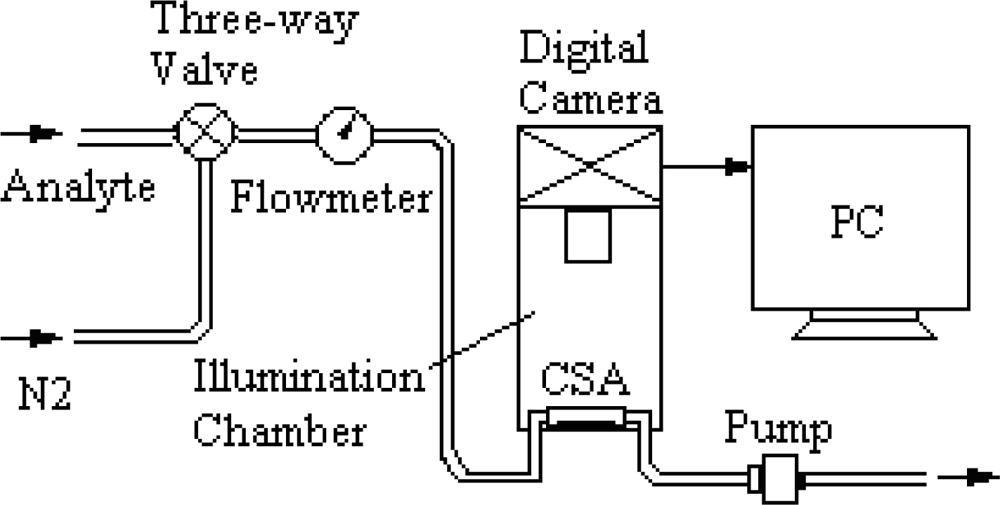
2.3. Experimental Setup
3. Results and Discussion
3.1. CSA’s Color Response to Analytes
3.2. Response Feature Extraction
3.3. Grouping of the Analytes
4. Conclusions
Abbreviations:
| CSA | Colorimetric sensor array |
| TMA | Trimethylamine |
| LDC | Linear discriminant criteria |
| PCA | Principle component analysis |
| HCA | Hierarchical cluster analysis |
| LOD | Limitation of detection |
| TPP | Tetraphenylporphine |
| OEP | Octaethylporphine |
| Pc | Phthalocyanine |
| SFS&SBS | a joint search algorithm of sequential forward selection and sequential backward selection |
Acknowledgments
References and Notes
- Wu, TH; Bechtel, PJ. Ammonia, dimehylamine, trimethylamine, and trimethylamine oxide from raw and processed fish by-products. J. Aquat. Food Prod. Technol 2008, 17, 27–38. [Google Scholar]
- Giovanna, S; Fausto, G. Biogenic amines in dry fermented sausages: a review. Int. J. Food Microbiol 2003, 88, 41–54. [Google Scholar]
- Bain, MA; Fauu, R; Fornasini, G; Milne, RW; Evans, AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Neph. Dia1. T 2006, 21, 1300–1304. [Google Scholar]
- Preti, G; Labows, JN; Kostelc, JG; Aldinger, S; Daniele, R. Analysis of lung air from patients with bronchogenic carcinoma and controls using gas chromatography-mass spectrometry. J. Chromatogr. B 1988, 432, 1–11. [Google Scholar]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem 2007, 103, 1475–1486. [Google Scholar]
- Chan, ST; Yao, MWY; Wong, YC; Wong, T; Mok, CS; Sin, DWM. Evaluation of chemica1 indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur. Food Res. Techno1 2006, 224, 67–74. [Google Scholar]
- Frank, R; Nicolae, B; Udo, W. Electronic nose: current status and future trends. Chem. Rev 2008, 108, 705–725. [Google Scholar]
- Hammond, J; Marquis, BB; Michaels, R; Oickle, B; Segee, B; Vetelino, J; Bushway, A; Camire, ME; Kathy, DD. A semiconducting metal-oxide array for monitoring fish freshness. Sens. Actuat. B 2002, 84, 113–122. [Google Scholar]
- Zhang, WH; Zhang, WD. Fabrication of SnO2–ZnO nanocomposite sensor for selective sensing of trimethylamine and the freshness of fishes. Sens. Actuat. B 2008, 134, 403–408. [Google Scholar]
- Cyrus, Z; Olga, C; Teresa, A; Morante, JR; Albert, RR. Detection of amines with chromium-doped WO3 mesoporous material. Sens. Actuat. B 2009, 140, 557–562. [Google Scholar]
- Ampuero, S; Bosset, JO. The electronic nose applied to dairy products: a review. Sens. Actuat. B 2003, 94, 1–12. [Google Scholar]
- Rakow, NA; Suslick, KS. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–714. [Google Scholar]
- John, C. Color and Constitution of Organic Molecules; Academic Press Inc (London) Ltd.: London, UK, 1976; pp. 33–45. [Google Scholar]
- Janzen, MC; Ponder, JB; Balley, DP; Ingison, CK; Suslick, KS. Colorimetric sensor arrays for volatile organic compounds. Anal. Chem 2006, 78, 3591–3600. [Google Scholar]
- Rakow, NA; Avijit, S; Michael, C; Ponder, JB; Suslick, KS. Molecular recognition and discrimination of amines with a colorimetric array. Angew. Chem. Int. Ed 2005, 44, 4528–4532. [Google Scholar]
- Lee, JH; Chang, BH; Kim, SD. Comparison of colour transformations for image segmentation. Electron. Lett 1994, 30, 1660–1661. [Google Scholar]
- Ricardo, GO; Nagle, HT. A method for evaluating data-preprocessing techniques for odor classification with an array of gas sensors. IEEE Trans. Syst. Man Cybern.-Part B: Cybern 1999, 29, 626–632. [Google Scholar]
- Spaulding, LD; Chang, CC; Yu, NT. Resonance raman spectra of metallooctaethylprophyrins. A structure probe of metal displacement. J. Am. Chem. Soc 1975, 97, 2517–2525. [Google Scholar]
- Hu, SQ; Jiang, TL. Study of the axial ligation of pyridine on metalloporphyrins and their UV-VIS spectra. Spectr. Sp. A 1992, 12, 33–37. [Google Scholar]
- Loris, N; Alessandra, L. Fusion of color spaces for ear authentication. Pattern Recognit 2009, 42, 1906–1913. [Google Scholar]
- Ohta, Y; Kanade, T; Sakai, T. Color information for region segmentation. Comput. Graph. Image Process 1980, 13, 222–241. [Google Scholar]
- Carmela, L; Severa, N; Lancet, D; Harel, D. An eNose algorithm for identifying chemicals and determining their concentration. Sens. Actuat. B 2003, 93, 77–83. [Google Scholar]







| cmpr(%) | JRGB | JXYZ | JL*a*b* | JYIQ | JHSV | JI1I2I3 | JMix | JFus |
|---|---|---|---|---|---|---|---|---|
| 0 | 22.984 | 47.833 | 18.974 | 26.609 | 10.897 | 26.477 | 21.731 | 238.68 |
| 2 | 38.07 | 110.92 | 25.964 | 48.996 | 13.673 | 42.645 | 35.321 | 434.73 |
| 4 | 59.058 | 123.78 | 41.467 | 83.127 | 16.981 | 79.431 | 73.28 | 476.62 |
| 6 | 119.55 | 147.35 | 70.534 | 138.83 | 21.45 | 151.77 | 120.34 | 515.95 |
| 8 | 119.55 | 161.01 | 122.54 | 138.83 | 27.287 | 151.77 | 158.92 | 515.95 |
| 10 | 161.37 | 161.01 | 122.54 | 138.83 | 36.217 | 151.77 | 190.17 | 515.95 |
© 2010 by the authors licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, Z.; Yang, J.; Yu, J.; Cui, B. A Colorimetric Sensor for Qualitative Discrimination and Quantitative Detection of Volatile Amines. Sensors 2010, 10, 6463-6476. https://doi.org/10.3390/s100706463
Tang Z, Yang J, Yu J, Cui B. A Colorimetric Sensor for Qualitative Discrimination and Quantitative Detection of Volatile Amines. Sensors. 2010; 10(7):6463-6476. https://doi.org/10.3390/s100706463
Chicago/Turabian StyleTang, Zhonglin, Jianhua Yang, Junyun Yu, and Bo Cui. 2010. "A Colorimetric Sensor for Qualitative Discrimination and Quantitative Detection of Volatile Amines" Sensors 10, no. 7: 6463-6476. https://doi.org/10.3390/s100706463




