Catalysts as Sensors—A Promising Novel Approach in Automotive Exhaust Gas Aftertreatment
Abstract
:1. Introduction
- ○ Current oxygen loading of three-way catalysts (TWC)
- ○ Current NOx-loading of lean NOx traps (LNT)
- ○ Current NH3-loading of ammonia-SCR catalysts (SCR)
- ○ Soot loading of Diesel particulate filters (DPF)
- ○ Conversion efficiency
- ○ Sulfur poisoning
- ○ And others.
- ○ In-situ monitoring of the impedance of ceria-zirconia based TWCs to determine their degree of oxygen loading,
- ○ In-situ measurement of the impedance of earth-alkaline oxide-based LNT coating materials to detect the status of an LNT with respect to its NOx-loading, its status of regeneration, its degree of sulfurization, and its thermal aging,
- ○ Approaches to determine the ammonia loading in Fe-SCR-zeolites with electrical ac measurements.
- ○ The oxygen loading degree of a TWC
- ○ The NOx-loading of an LNT and
- ○ The soot loading of a DPF.
2. Background
3. Impedance-Based Direct Catalyst Diagnosis
3.1. Three-Way Catalyst (TWC)
3.2. Lean NOx Trap (LNT)
3.3. Ammonia-SCR-Catalyst
4. Radio Frequency-Based Contactless Direct Catalyst Diagnosis
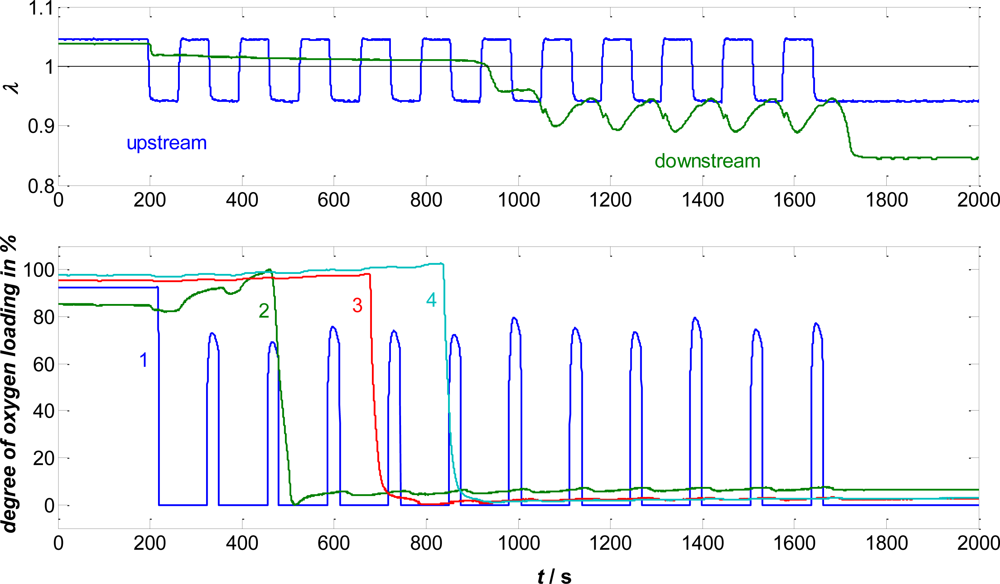
4.1. Oxygen Loading of a TWC
4.2. NOx-loading of LNTs
4.3. Soot Loading of DPFs
5. Conclusions and Outlook
Acknowledgments
References
- Riegel, J; Neumann, H; Wiedenmann, H-M. Exhaust gas sensors for automotive emission control. Solid State Ionics 2002, 152–153, 783–800. [Google Scholar]
- Moos, R. A brief overview on automotive exhaust gas sensors based on electroceramics. Int. J. Appl. Ceram. Technol 2005, 2, 401–413. [Google Scholar]
- Zhuiykov, S; Miura, N. Development of zirconia-based potentiometric NOx sensors for automotive and energy industries in the early 21st century: What are the prospects for sensors? Sens. Actuator. B Chem 2007, 121, 639–651. [Google Scholar]
- Fergus, JW. Solid electrolyte based sensors for the measurement of CO and hydrocarbon gases. Sens. Actuator. B Chem 2007, 122, 683–693. [Google Scholar]
- Moos, R; Schönauer, D. Review: Recent developments in the field of automotive exhaust gas ammonia sensing. Sens. Lett 2008, 6, 821–825. [Google Scholar]
- Alkemade, UG; Schumann, B. Engines and exhaust after treatment systems for future automotive applications. Solid State Ionics 2006, 177, 2291–2296. [Google Scholar]
- Shelef, M; McCabe, RW. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar]
- Koebel, M; Elsener, M; Kröcher, O; Schär, C; Röthlisberger, R; Jaussi, F; Mangold, M. NOx reduction in the exhaust of mobile heavy-duty diesel engines by urea-SCR. Topics Catal 2004, 43, 30–31. [Google Scholar]
- Busca, G; Lietti, L; Ramis, G; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ 1998, 18, 1–36. [Google Scholar]
- Kröcher, O; Devadas, M; Elsener, M; Wokaun, A; Söger, N; Pfeifer, M; Demel, Y; Mussmann, L. Investigation of the selective catalytic reduction of NO by NH3 on Fe-ZSM5 monolith catalysts. Appl. Catal. B Environ 2006, 66, 208–216. [Google Scholar]
- Takeuchi, M; Matsumoto, S. NOx storage-reduction catalysts for gasoline engines. Topics Catal 2004, 28, 151–156. [Google Scholar]
- Rohr, F; Göbel, U; Kattwinkel, P; Kreuzer, T; Müller, W; Philipp, S; Gélin, P. New insight into the interaction of sulfur with diesel NOx storage catalysts. Appl. Catal. B Environ 2007, 70, 189–197. [Google Scholar]
- Twigg, MV; Phillips, PR. Cleaning the air we breathe—Controlling diesel particulate emissions from passenger cars. Platinum Met. Rev 2009, 53, 27–34. [Google Scholar]
- Möller, R; Votsmeier, M; Onder, C; Guzzella, L; Gieshoff, J. Is oxygen storage in three-way catalysts an equilibrium controlled process? Appl. Catal. B Environ 2009, 91, 30–38. [Google Scholar]
- Tuller, HL; Nowick, AS. Defect structure and electrical properties of nonstoichiometric CeO2 single crystals. J. Electrochem. Soc 1979, 126, 209–217. [Google Scholar]
- Izu, N; Oh-hori, N; Shin, W; Matsubara, Í; Murayama, N; Itou, M. Response properties of resistive oxygen sensors using Ce1−xZrxO2 (x = 0.05, 0.10) thick films in propane combustion gas. Sens. Actuator. B Chem 2008, 130, 105–109. [Google Scholar]
- Reiß, S; Wedemann, M; Moos, R; Rösch, M. Electrical in situ characterization of three-way catalyst coatings. Topics Catal 2009, 52, 1898–1902. [Google Scholar]
- Reiß, S; Spörl, M; Hagen, G; Fischerauer, G; Moos, R. Combination of wirebound and microwave measurements for in-situ characterization of automotive three-way catalysts. IEEE Sens J 2010. [Google Scholar] [CrossRef]
- Tuttlies, U; Schmeißer, V; Eigenberger, G. A new simulation model for NOx storage catalyst dynamics. Topics Catal 2004, 30–31, 187–192. [Google Scholar]
- Moos, R; Zimmermann, C; Birkhofer, T; Knezevic, A; Plog, C; Busch, MR; Ried, T. Sensor for directly determining the state of a NOx storage catalyst. SAE Paper 2008. 2008-01-0447. [Google Scholar]
- Zimmermann, C. Neuartiger Sensor zur Bestimmung des Zustandes eines NOx-Speicher-katalysators (Novel sensor for determining the state of a NOx storage catalyst). Ph.D. thesis; University of Bayreuth: Bayreuth, Germany, 2007. [Google Scholar]
- Geupel, A; Schönauer, D; Röder-Roith, U; Kubinski, DJ; Mulla, S; Ballinger, TH; Chen, H-Y; Visser, JH; Moos, R. Integrating nitrogen oxide sensor: A novel concept for measuring low concentrations in the exhaust gas. Sens. Actuator. B Chem 2010, 145, 756–761. [Google Scholar]
- Moos, R; Wedemann, M; Spörl, M; Reiß, S; Fischerauer, G. Direct catalyst monitoring by electrical means: An overview on promising novel principles. Topics Catal 2009, 52, 2035–2040. [Google Scholar]
- Traebert, A; Zimmermann, L; Frey, R; Johansson, T. System layout and DeNOx-Performance of a combined after-treatment-system for commercial vehicles—simulation study and test bench investigations. Proceedings of the 6th International Exhaust Gas and Particulate Emissions Forum, Ludwigsburg, Germany, 9–10 March 2010; pp. 161–168.
- Hsieh, MF; Wang, J. Nonlinear observer designs for diesel engine selective catalytic reduction (SCR) ammonia coverage ratio estimation. Proceedings of Decision and Control, 2009 held jointly with the 2009 28th Chinese Control Conference. CDC/CCC, Shanghai, China, 16–18 December 2009. [CrossRef]
- Kubinski, DJ; Visser, J. Sensor and method for determining the ammonia loading of a zeolite SCR catalyst. Sens. Actuator. B Chem 2008, 130, 425–429. [Google Scholar]
- Simon, U; Flesch, U; Maunz, W; Müller, R; Plog, C. The effect of NH3 on the ionic conductivity of dehydrated zeolites Na beta and H beta. Microporous Mesoporous Mater 1998, 21, 111–116. [Google Scholar]
- Moos, R; Müller, R; Plog, C; Knezevic, A; Leye, H; Irion, E; Braun, T; Marquardt, K; Binder, K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuator. B Chem 2002, 83, 181–189. [Google Scholar]
- Franke, M; Simon, U; Moos, R; Knezevic, A; Müller, R; Plog, C. Development and working principle of an ammonia gas sensor based on a refined model for solvate supported proton transport in zeolites. Phys. Chem. Chem. Phys 2003, 5, 5195–5198. [Google Scholar]
- Rodríguez-González, L; Simon, U. NH3-TPD measurements using a zeolite-based sensor. Meas. Sci. Technol 2010, 21, 027003. [Google Scholar]
- Boaro, M; Trovarelli, A; Hwang, JH; Mason, TO. Electrical and oxygen storage/release properties of nanocrystalline ceria-zirconia solid solutions. Solid State Ionics 2002, 147, 85–95. [Google Scholar]
- Fischerauer, G; Spörl, M; Gollwitzer, A; Wedemann, M; Moos, R. Catalyst state observation via the perturbation of a microwave cavity resonator. Frequenz 2008, 62, 180–184. [Google Scholar]
- Moos, R; Spörl, M; Hagen, G; Gollwitzer, A; Wedemann, M; Fischerauer, G. TWC: Lambda control and OBD without lambda probe—an initial approach. SAE Papers 2008. 2008-01-0916. [Google Scholar]
- Saji, K; Kondo, H; Takeuchi, T; Igarashi, I. Voltage step characteristics of oxygen concentration cell sensors for nonequilibrium gas mixtures. J. Electrochem. Soc 1988, 137, 1686–1691. [Google Scholar]
- Reiß, S; Wedemann, M; Spörl, M; Fischerauer, G; Moos, R. Effects of H2O, CO2, CO, and flow rates on the RF-based monitoring of three-way catalysts. Sens Lett 2009. Submitted. [Google Scholar]
- Fino, D. Diesel emission control: Catalytic filters for particulate removal. Sci. Technol. Adv. Mater 2007, 8, 93–100. [Google Scholar]
- Johnson, TV. Diesel emission control in review. SAE Papers 2007. 2007-01-0233. [Google Scholar]
- Riegel, J; Klett, S. Sensors for modern exhaust gas after-treatment systems. Proceedings of the 5th International Exhaust Gas and Particulate Emissions Forum, Ludwigsburg, Germany, 19–20 February 2008; pp. 84–97.
- Weigel, M; Roduner, C; Lauer, T. Particle-filter onboard-diagnosis by means of a soot-sensor downstream of the particle-filter. Proceedings of the 6th International Exhaust Gas and Particulate Emissions Forum, Ludwigsburg, Germany, 9–10 March 2010; pp. 62–69.
- Hagen, G; Feistkorn, C; Wiegärtner, S; Heinrich, A; Brüggemann, D; Moos, R. Conductometric soot sensor for automotive exhausts: Initial studies. Sensors 2010, 10, 1589–1598. [Google Scholar]
- Ochs, T; Schittenhelm, H; Genssle, A; Kamp, B. Particulate matter sensor for on board diagnostics (obd) of diesel particulate filters (DPF). SAE Papers 2010. 2010-01-0307. [Google Scholar]
- Müller, N; Moos, R; Jess, A. In situ monitoring of coke deposits during coking and regeneration of solid catalysts by electrical impedance-based sensors. Chem. Eng. Technol 2010, 33, 103–112. [Google Scholar]
- Fischerauer, G; Förster, M; Moos, R. Sensing the soot load in automotive diesel particulate filters by microwave methods. Meas. Sci. Technol 2010, 21, 035108. [Google Scholar]







© 2010 by the authors licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moos, R. Catalysts as Sensors—A Promising Novel Approach in Automotive Exhaust Gas Aftertreatment. Sensors 2010, 10, 6773-6787. https://doi.org/10.3390/s100706773
Moos R. Catalysts as Sensors—A Promising Novel Approach in Automotive Exhaust Gas Aftertreatment. Sensors. 2010; 10(7):6773-6787. https://doi.org/10.3390/s100706773
Chicago/Turabian StyleMoos, Ralf. 2010. "Catalysts as Sensors—A Promising Novel Approach in Automotive Exhaust Gas Aftertreatment" Sensors 10, no. 7: 6773-6787. https://doi.org/10.3390/s100706773





