Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review
Abstract
:1. Introduction
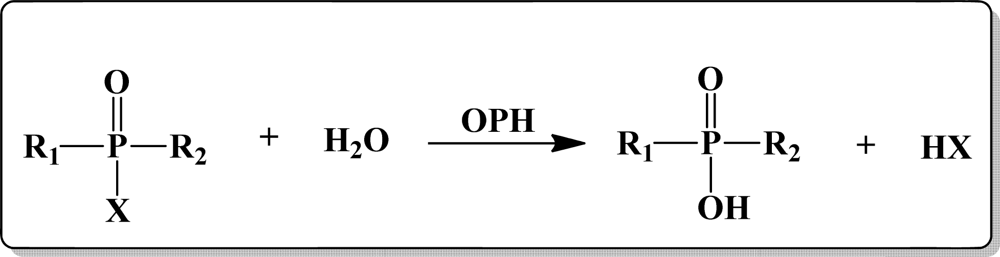
1.1. Structure of Organophosphorus Compounds
1.2. OP Compounds and Their Toxicity
2. Advances in Detection of OP compounds
2.1. Fluorescence-based Biosensors for OP Compounds
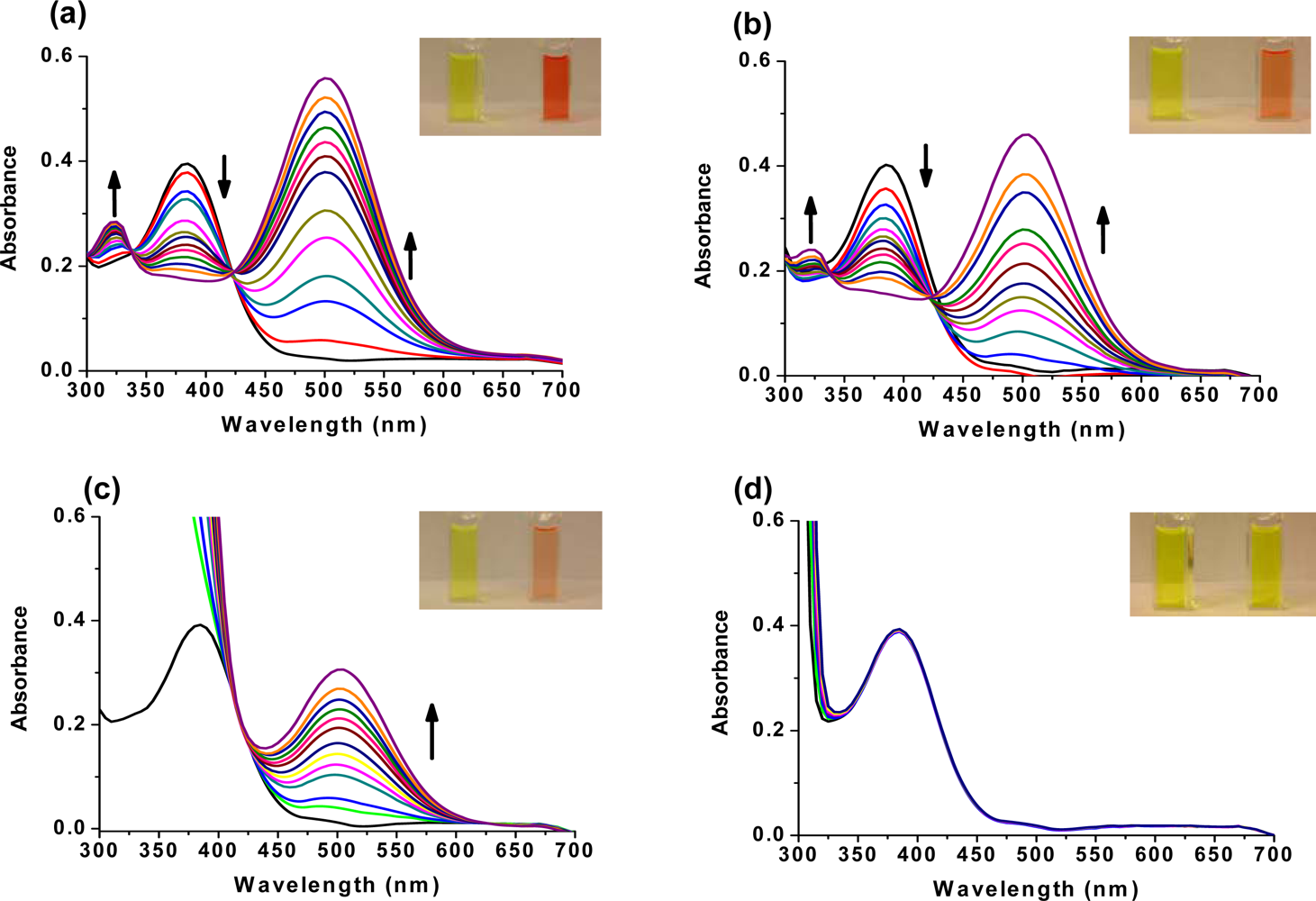
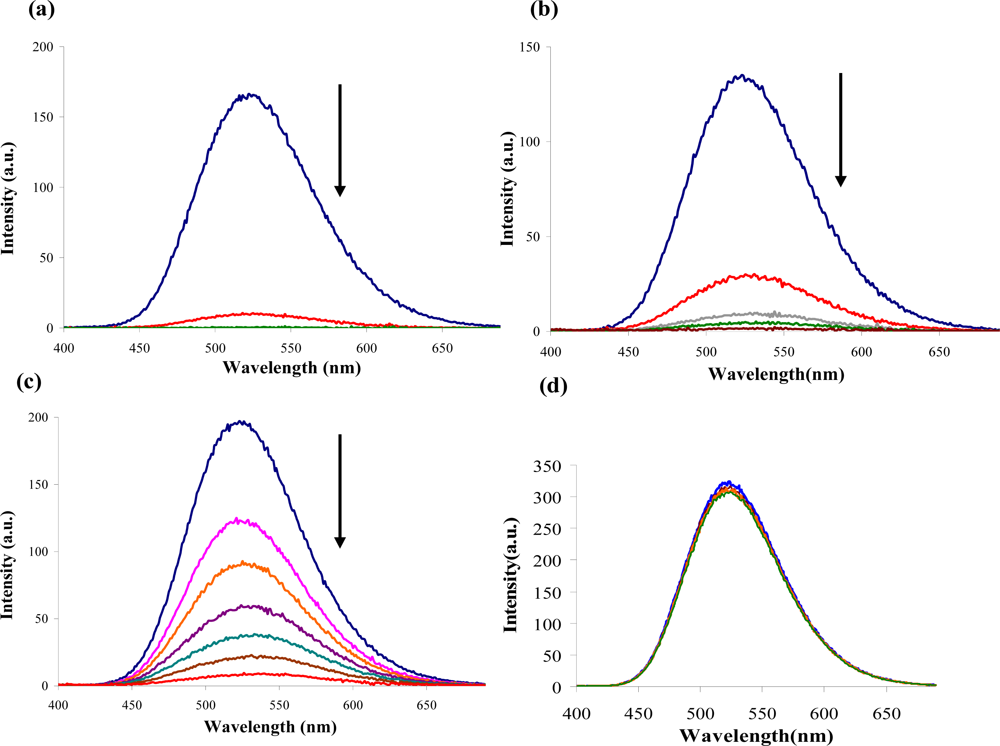
2.2. Fluorescence-based Chemosensor Detection Methods
3. Sensors with Multiple Modes of Signal Transduction
4. Future Perspectives
Acknowledgments
References
- Ariese, F; Ernst, WHO; Sijm, DTHM. Natural and synthetic organic compounds in the environment—A symposium report. Environ. Toxicol. Pharmacol 2001, 10, 65–80. [Google Scholar]
- Karr, JR; Dudley, DR. Ecological perspective on water quality goals. Environ. Manage 1981, 5, 55–68. [Google Scholar]
- U.S. EPA. Pesticides and food: Why children may be especially sensitive to pesticides. Available online: http://www.epa.gov/pesticides/food/pest.htm (accessed on February 26, 2010).
- Survey, U.S.G. Organophosphorus pesticides occurrence and distribution in surface and ground water of the United States. Available online: http://ga.water.usgs.gov/publications/ofr00-187.pdf. (accessed on 26 February 2010).
- The Pesticide Action Network (PAN). Pesticide Action Network (PAN) pesticide database. Available online: http://www.pesticideinfo.org. (accessed on 26 February 2010).
- International Programme on Chemical Safety (INCHEM). International Programme on Chemical Safety INCHEM Database. Available online: http://www.inchem.org (accessed on 26 February 2010).
- Ullmann's Agrochemicals; Wiley-VCH: Weinheim, Germany, 26 March 2007; Volume 1.
- Celik, S; Kunc, S; Asan, T. Degradation of some pesticides in the field and effect of processing. Analyst 1995, 120, 1739–1743. [Google Scholar]
- Bouchard, MF; Bellinger, DC; Wright, RO; Weisskopf, MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 2010, 125, 1216–1226. [Google Scholar]
- Liu, GL; Lin, Y. Electrochemical sensor for organophosphate pesticides and nerve agents using Zirconia nanoparticles as selective Sorbents. Anal. Chem 1995, 77, 5894–5901. [Google Scholar]
- Gilliom, RJB; Barbash, JE; Kolpin, DW; Larson, SJ. Testing water quality for pesticide pollution. Environ. Sci. Technol 1999, 33, 164A–169A. [Google Scholar]
- Vermeire, T; MacPhail, R; Waters, M. Integrated human and ecological risk assessment: A case study of organophosphorous pesticides in the environment. Hum. Ecol. Risk. Assessment 2003, 9, 343–357. [Google Scholar]
- Walker, BJ. Organophosphorus Chemistry; Penguin: London, UK, 1972. [Google Scholar]
- Jenkins, AL; Uy, OM; Murray, GM. Polymer based lanthanide luminescent sensors for the detection of nerve agents. Anal. Commun 1997, 34, 221–224. [Google Scholar]
- Jenkins, AL; Uy, OM; Murray, GM. Polymer-based lanthanide luminescent sensor for detection of the hydrolysis product of the nerve agent Soman in water. Anal. Chem 1999, 71, 373–378. [Google Scholar]
- Jenkins, AL; Yin, R; Jensen, JL. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Analyst 2001, 126, 798–802. [Google Scholar]
- Rudzinski, CM; Young, AM; Nocera, DG. A supramolecular microfluidic optical chemosensor. J. Am. Chem. Soc 2002, 124, 1723–1727. [Google Scholar]
- Russell, AJ; Berberich, JA; Drevon, GE; Koepsel, RR. Biomaterials for mediation of chemical and biological warfare agents. Annu. Rev. Biomed. Eng 2003, 5, 1–27. [Google Scholar]
- Sohn, H; Letant, S; Sailor, MJ; Trogler, WC. Detection of fluorophosphonate chemical warfare agents by catalytic hydrolysis with a porous silicon interferometer. J. Am. Chem. Soc 2000, 122, 5399–5400. [Google Scholar]
- Steiner, WE; Klopsch, SJ; English, WA; Clowers, BH; Hill, HH. Detection of a chemical warfare agent simulant in various aerosol matrixes by ion mobility time-of-flight mass spectrometry. Anal. Chem 2005, 77, 4792–4799. [Google Scholar]
- Ross, RT; Biros, FJ. Correlations between 31P NMR chemical shifts and structures of some organophosphorus pesticides. Anal. Chim. Acta 1970, 52, 139–141. [Google Scholar]
- Michel, HO; Gordon, EC; Epstein, J. Detection and estimation of isopropyl methylphosphonofluoridate and O-ethyl S-diisopropylaminoethyl methylphosphonothioate in seawater in parts-per-trillion level. Environ. Sci. Technol 1973, 7, 1045–1049. [Google Scholar]
- Novak, TJ; Daasch, LW; Epstein, J. Decomposition at 90 degrees celcius of the cholinesterase substrate indoxyl acetate impregnated on paper supports. Anal. Chem 1979, 51, 1271–1275. [Google Scholar]
- Wallace, KJ; Morey, J; Lynch, VM; Anslyn, EV. Colorimetric detection of chemical warfare simulants. New J. Chem 2005, 29, 1469–1474. [Google Scholar]
- Ngehngwainbi, J; Foley, PH; Kuan, SS; Guilbault, GG. Parathion antibodies on piezoelectric crystals. J. Am. Chem. Soc 1986, 108, 5444–5447. [Google Scholar]
- Nieuwenhuizen, MS; Harteveld, JLN. Studies on a surface acoustic wave (SAW) dosimeter sensor for organophosphorous nerve agents. Sens. Actuat. B 1997, 40, 167–173. [Google Scholar]
- Yamaguchi, S; Yoshimura, L; Kohira, T; Tamaru, S; Hamachi, I. Cooperation between artificial receptors and supramolecular hydrogels for sensing and discriminating phosphate derivatives. J. Am. Chem. Soc 2005, 127, 11835–11841. [Google Scholar]
- Zhang, SW; Swager, TM. Fluorescent detection of chemical warfare agents: Functional group specific ratiometric chemosensors. J. Am. Chem. Soc 2003, 125, 3420–3421. [Google Scholar]
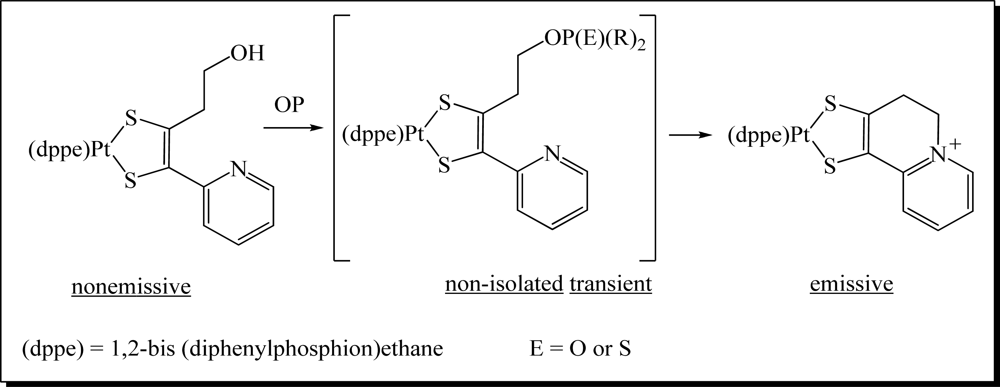
- Van Houten, KA; Heath, DC; Pilato, RS. Rapid luminescent detection of phosphate esters in solution and the gas phase using (dppe)Pt{S2C2(2-pyridyl)(CH2CH2OH)}. J. Am. Chem. Soc 1998, 120, 12359–12360. [Google Scholar]
- Evtugyn, GA; Budnikov, HC; Nikolskaya, EB. Influence of surface-active compounds on the response and sensitivity of cholinesterase biosensors for inhibitor determination. Analyst 1996, 121, 1911–1915. [Google Scholar]
- Sherma, J. Pesticides. Anal. Chem 1995, 67, R1–R20. [Google Scholar]
- Trojanowicz, M. Determination of pesticides using electrochemical enzymatic biosensors. Electroanalysis 2002, 14, 1311–1328. [Google Scholar]
- Martínez-Máñez, R; Sancenón, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev 2003, 103, 4419–4476. [Google Scholar]
- Brufani, M; Marta, M; Pomponi, M. Anticholinesterase activity of a new carbamate, heptylphysostigmine, in view of its use in patients with Alzheimer-type dementia. Eur. J. Biochem 1986, 157, 115–120. [Google Scholar]
- Lei, Y; Mulchandani, P; Wang, J; Chen, W; Mulchandani, A. Highly sensitive and selective amperometric microbial biosensor for direct determination of p-nitrophenyl-substituted organophosphate nerve agents. Environ. Sci. Technol 2005, 39, 8853–8857. [Google Scholar]
- Mionetto, N; Marty, JL; Karube, I. Acetylcholinesterase in organic-solvents for the detection of pesticides—Biosensor application. Biosens. Bioelectr 1994, 9, 463–470. [Google Scholar]
- Palleschi, G; Bernabei, M; Cremisini, C; Mascini, N. Determination of organophosphorus insecticides with a choline electrochemical biosensor. Sens. Actuat. B 1992, 7, 513–517. [Google Scholar]
- Cao, X; Mello, SV; Leblanc, RM; Rastogi, VK; Cheng, TC; DeFrank, JJ. Detection of paraoxon by immobilized organophosphorus hydrolase in a langmuir-blodgett film. Colloids Surf. A 2004, 250, 349–356. [Google Scholar]
- Rudzinski, CMY; Young, AM; Nocera, DG. Supramolecular Microfluidic Optical Chemosensor. J. Am. Chem. Soc 2002, 124, 1723–1727. [Google Scholar]
- Burnworth, M; Rowan, SJ; Weder, C. Fluorescent Sensors for the detection of chemical warfare agents. Chem. Eur. J 2007, 13, 7828–7836. [Google Scholar]
- Rogers, KR; Cao, CJ; Valdes, JJ; Elderfrawi, AT; Eldefrai, ME. Acetylcholinesterase fiber-optic biosensor for detection of anticholinesterases. Appl. Toxicol 1991, 16, 810–820. [Google Scholar]
- Cao, H; Nam, J; Harmon, HJ; Branson, DH. Spectrophotometric detection of organophosphate diazinon by porphyrin solution and porphyrin-dyed cotton fabric. Dyes Pigments 2007, 74, 176–180. [Google Scholar]
- Dave, KI; Miller, CE; Wild, JR. Characterization of organophosphorus hydrolases and the genetic manipulation of the phosphotriesterase from Pseudomonas diminuta. Chem-Biol. Interact 1993, 87, 55–68. [Google Scholar]
- Ma, LY; Wang, HY; Xie, H; Xu, LX. A long lifetime chemical sensor: study on fluorescence property of fluorescein isothiocyanate and preparation of pH chemical sensor. Spectrochim. Acta A 2004, 60, 1865–1872. [Google Scholar]
- Kolosova, AY; Park, JH; Eremin, SA; Kang, SJ; Chung, D. Fluorescence polarization immunoassay based on a monoclonal antibody for the detection of the organophosphorus pesticide parathion-methyl. J. Agric. Food Chem 2003, 51, 1107–1114. [Google Scholar]
- Kolosova, AY; Park, JH; Eremin, SA; Park, SJ; Kang, SJ; Lee, HS; Chung, DH. Comparative study of three mmunoassays based on monoclonal antibodies for detection of the pesticide parathion-methyl in real samples. Anal. Chim. Acta 2004, 511, 323–331. [Google Scholar]
- Lee, EK; Kim, YJ; Park, WC; Chung, TW; Lee, YT. Monoclonal antibody-based enzyme-linked immunosorbent assays for the detection of the organophosphorus insecticide diazinon. Anal. Chim. Acta 2005, 530, 143–153. [Google Scholar]
- Tang, SJ; Zhang, M; Cheng, CG; Lu, YT. Development of fluorescence polarization immunoassay for the detection of the organophosphorus pesticides parathion and azinophos-methyl. J. Immuno. Immunochem 2008, 29, 356–369. [Google Scholar]
- Zhang, SW; Swager, TM. Fluorescent detection of chemical warfare agents: Functional group specific ratiometric chemosensors. J. Am. Chem. Soc 2003, 125, 3420–3421. [Google Scholar]
- Dale, TJ; Rebek, J. Fluorescent sensors for organophosphorus nerve agent mimics. J. Am. Chem. Soc 2006, 128, 4500–4501. [Google Scholar]
- Paliwal, S; Wales, M; Good, T; Grimsley, J; Wild, J; Simonian, A. Fluorescence-based sensing of p-nitrophenol and p-nitrophenyl substituent organophosphates. Anal. Chim. Acta 2007, 596, 9–15. [Google Scholar]
- Delattre, F; Cazier, F; Cazier, F; Tine, A. Use a fluorescent molecular sensor for the detection of pesticides and herbicides in water. Curr. Anal. Chem 2009, 5, 48–52. [Google Scholar]
- Sun, XY; Xia, KH; Liu, B. Design of fluorescent self-assembled multilayers and interfacial sensing for organophosphorus pesticides. Talanta 2008, 76, 747–751. [Google Scholar]
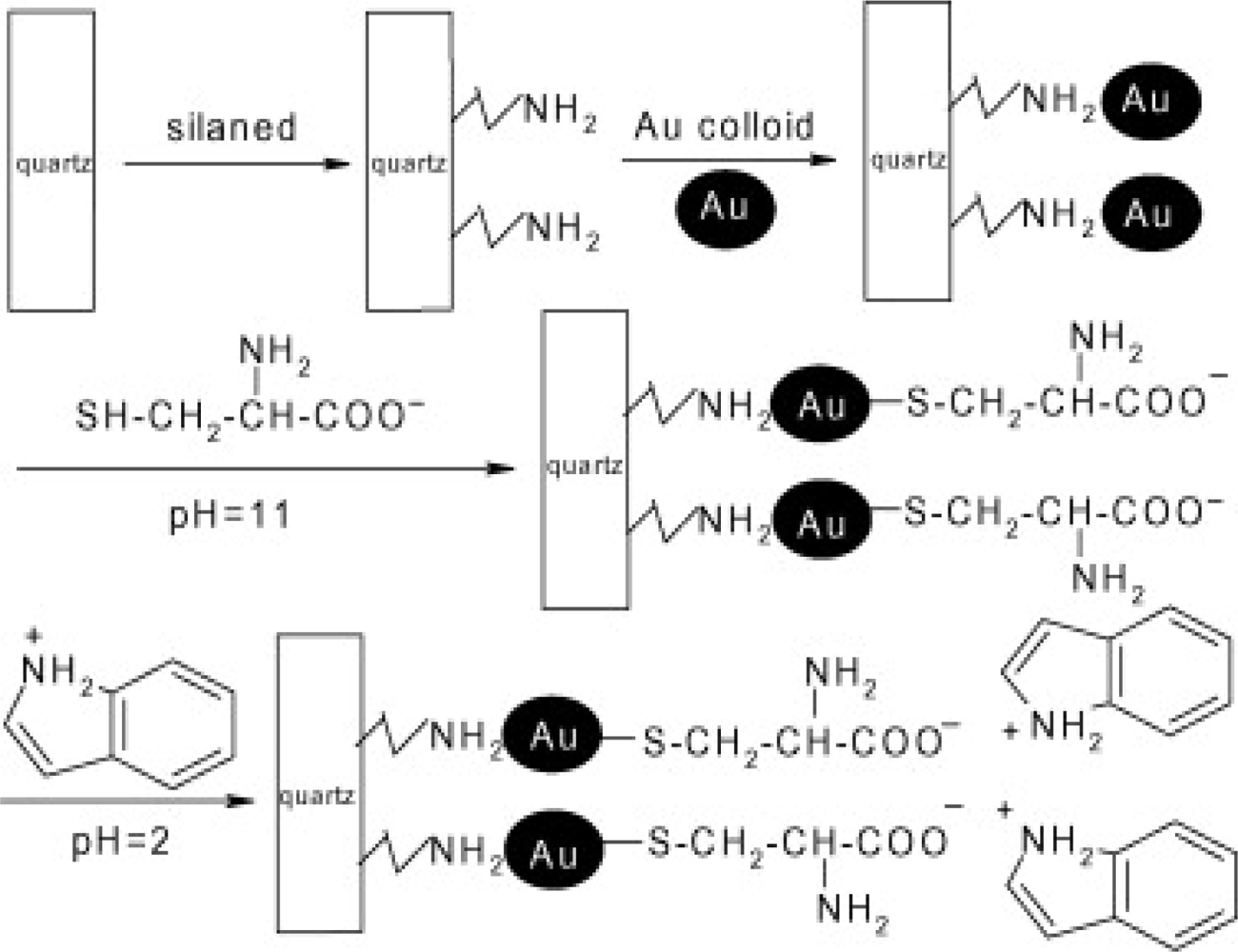
- Beaudoin, DS; Obare, SO. Dual optical and electrochemical saccharide detection based on a dipyrido[3,2-a:2′3′-c]phenazine (DPPZ) ligand. Tetrahedron Lett 2008, 49, 6054–6057. [Google Scholar]
- De, C; Samuels, TA; Haywood, TL; Anderson, GA; Campbell, K; Fletcher, K; Murray, DH; Obare, SO. Dual colorimetric and electrochemical sensing of organothiophosphorus pesticides by an azastilbene derivative. Tetrahedron Lett 2010, 51, 1754–1757. [Google Scholar]
- Wheeler, OH; Batlle de Pabon, HN. Synthesis of stilbenes. A comparative study. J. Org. Chem 1965, 30, 1473–1477. [Google Scholar]
- Becker, KB. Synthesis of stilbenes. Synthesis 1983, 5, 341–368. [Google Scholar]
- Takahashi, K; Okamoto, T; Yamada, K; Iida, H. A Convenient synthesis of substituted stilbenes by condensation of o- or p-Tolunitrile with p-Substituted benzaldehydes. Synthesis 1977, 1, 58. [Google Scholar]
- Wang, SL; Ho, TI. Protonation dependent electron transfer in 2-styrylquinolines. Chem. Phys. Lett 1997, 268, 434–438. [Google Scholar]
- Ko, HJ; Park, TH. Dual signal transduction mediated by a single type of olfactory receptor expressed in a heterologous system. Biol. Chem 2006, 387, 59–68. [Google Scholar]
- Allen, KN; Dunaway-Mariano, D. Phosphoryl group transfer: Evolution of a catalytic scaffold. Trends Biochem. Sci 2004, 29, 495–503. [Google Scholar]
- Frisch, MJ; Trucks, GW; Schlegel, HB; Scuseria, GE; Robb, MA; Cheeseman, JR; Montgomery, JA, Jr; Vreven, TV; Kudin, KN; Burant, JC; Millam, JM; Iyengar, SS; Tomasi, J; Barone, V; Mennucci, B; Cossi, M; Scalmani, G; Rega, N; Petersson, GA; Nakatsuji, H; Hada, M; Ehara, M; Toyota, K; Fukuda, R; Hasegawa, J; Ishida, M; Nakajima, T; Honda, Y; Kitao, O; Nakai, H; Klene, M; Li, X; Knox, JE; Hratchian, HP; Cross, JB; Bakken, V; Adamo, C; Jaramillo, J; Gomperts, R; Stratmann, RE; Yazyev, O; Austin, AJ; Cammi, R; Pomelli, C; Ochterski, JW; Ayala, PY; Morokuma, K; Voth, GA; Salvador, P; Dannenberg, JJ; Zakrzewski, VG; Dapprich, S; Daniels, AD; Strain, MC; Farkas, O; Malick, DK; Rabuck, AD; Raghavachari, K; Foresman, JB; Ortiz, JV; Cui, Q; Baboul, AG; Clifford, S; Cioslowski, J; Stefanov, BB; Liu, G; Liashenko, A; Piskorz, P; Komaromi, I; Martin, RL; Fox, DJ; Keith, T; Al-Laham, MA; Peng, CY; Nanayakkara, A; Challacombe, M; Gill, PMW; Johnson, B; Chen, W; Wong, MW; Gonzalez, C; Pople, JA. Gaussian 03; Gaussian, Inc: Wallingford CT, USA, 2004. [Google Scholar]










| No | OP Name | Structure (Thions: 1–17; Oxons: 18–29) | LD50, mg/kg * | WHO Acute Hazard§ | IARC Carcinogens‡ | U.S. EPA Carcinogens† | |
|---|---|---|---|---|---|---|---|
| Oral | Dermal | ||||||
| 1 | Parathion |  | 1 | 21 | Ia | 3, Unclassifiable | C, Possible |
| 2 | Fonofos |  | 8–17 | 147 | Ia | N/A | E, Unlikely |
| 3 | Azinphos-methyl |  | 11–13 | 220 | Ib | N/A | Not Likely |
| 4 | Coumaphos | 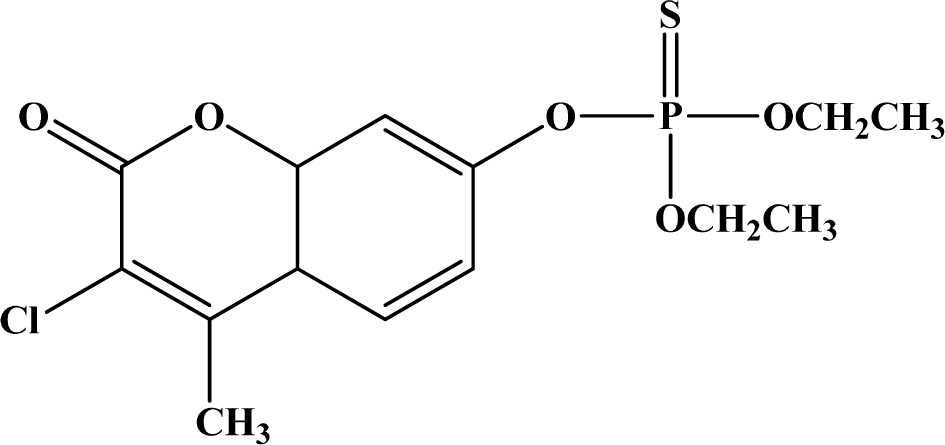 | 16–41 | 1,000 | Ib | N/A | Not Likely |
| 5 | Methidathion |  | 25–48 | 1,546 | Ib | N/A | C, Possible |
| 6 | Leptophos |  | 45–53 | >800 | N/A | N/A | N/A |
| 7 | Propetamphos | 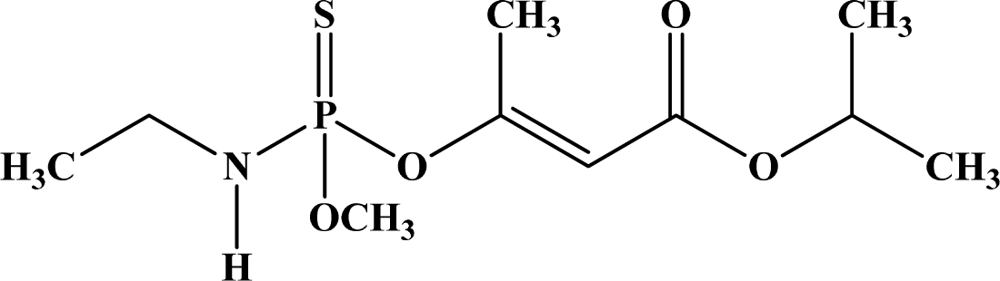 | 75–82 | 2,300 | Ib | N/A | Not Likely |
| 8 | Carbophenothion | 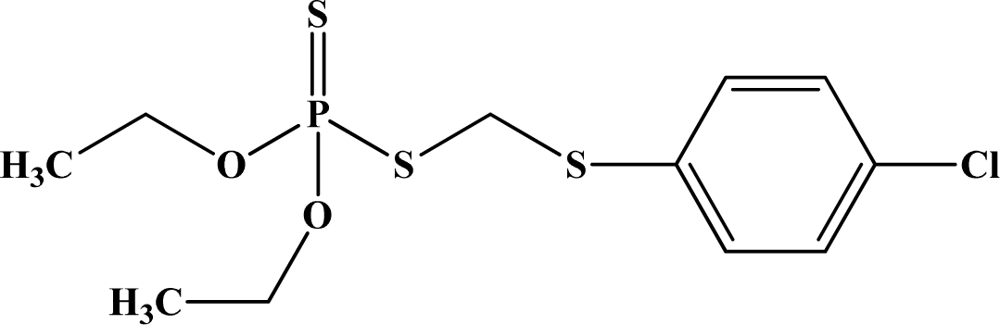 | 98–120 | 190–215 | N/A | N/A | N/A |
| 9 | Phosmet |  | 113–160 | >1,500 | II | N/A | Suggestive |
| 10 | Chlorpyrifos | 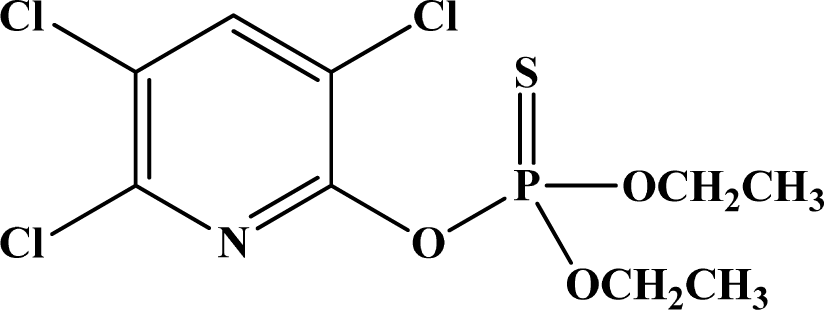 | 135–165 | 2,000 | II | N/A | E, Unlikely |
| 11 | Fenthion |  | 214–245 | 330 | II | N/A | E, unlikely |
| 12 | Fenitrothion | 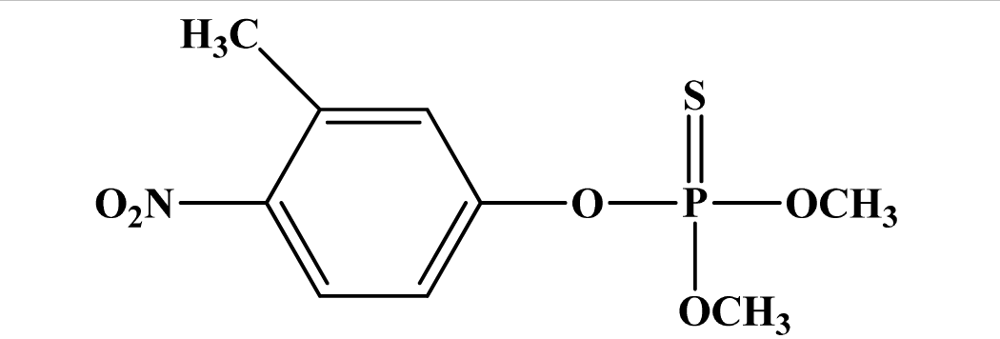 | 250 | >3,000 | II | N/A | E, unlikely |
| 13 | Dichlofenthion |  | 270 | 6,000 | N/A | N/A | N/A |
| 14 | Dicapthon |  | 330–400 | 790–1,250 | N/A | N/A | N/A |
| 15 | Diazinon |  | 300–850 | 2,150 | II | N/A | Not Likely |
| 16 | Ronnel |  | 1,250–2,630 | 2,000 | N/A | N/A | N/A |
| 17 | Malathion | 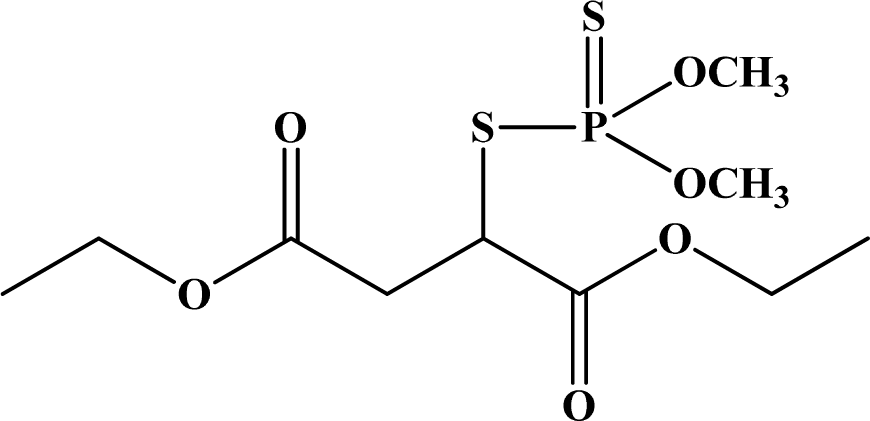 | 5,400–5,700 | >2,000 | III | 3, Unclassifiable | Suggestive |
| 18 | Tetraethyl pyrophosphate (TEPP) |  | 0.5 | 2.4 | N/A | N/A | N/A |
| 19 | Mevinphos |  | 3.7–6.1 | 4.2–2.7 | Ia | N/A | N/A |
| 20 | Schradan | 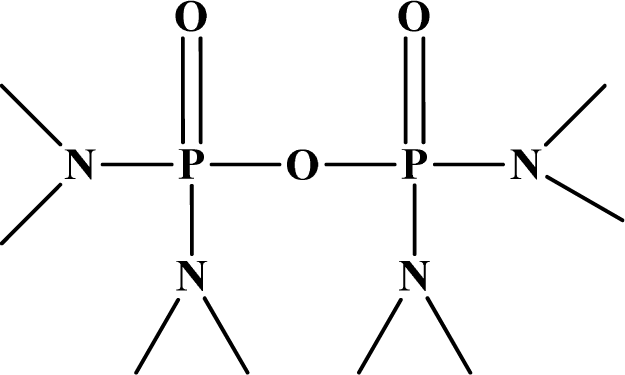 | 10 | 15 | N/A | N/A | N/A |
| 21 | Monocrotophos |  | 18–20 | 112–126 | Ib | N/A | N/A |
| 22 | Phosphamidon |  | 24 | 107–143 | Ia | N/A | C, Possible |
| 23 | Oxydemeton methyl |  | 47–52 | 158–173 | Ib | N/A | Not Likely |
| 24 | Ethoprophos | 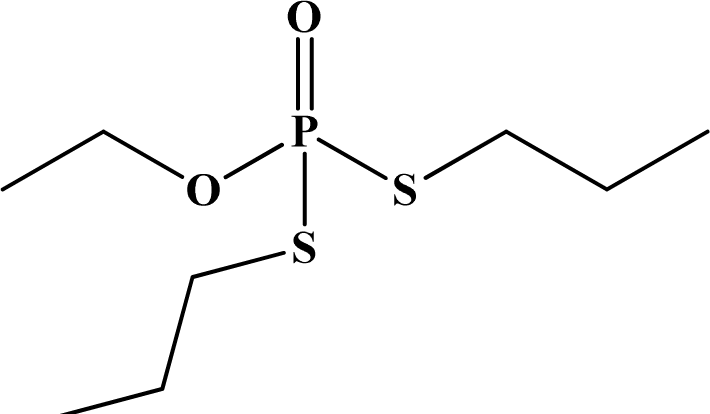 | 61 | 26 | Ia | N/A | Likely |
| 25 | Dichlorvos |  | 56–80 | 75–107 | Ib | 2b, Possible | Suggestive |
| 26 | Crotoxyphos |  | 74–110 | 202–375 | N/A | N/A | N/A |
| 27 | Naled |  | 250 | 800 | II | N/A | E, Unlikely |
| 28 | Tribufos |  | 560–630 | >2,000 | N/A | N/A | Likely (high doses), Not likely (low doses) |
| 29 | Trichlorfon |  | 400–800 | >2,000 | II | 3, Unclassifiable | Likely (high doses), Not likely (low doses) |
© 2010 by the authors licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018-7043. https://doi.org/10.3390/s100707018
Obare SO, De C, Guo W, Haywood TL, Samuels TA, Adams CP, Masika NO, Murray DH, Anderson GA, Campbell K, et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors. 2010; 10(7):7018-7043. https://doi.org/10.3390/s100707018
Chicago/Turabian StyleObare, Sherine O., Chandrima De, Wen Guo, Tajay L. Haywood, Tova A. Samuels, Clara P. Adams, Noah O. Masika, Desmond H. Murray, Ginger A. Anderson, Keith Campbell, and et al. 2010. "Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review" Sensors 10, no. 7: 7018-7043. https://doi.org/10.3390/s100707018





