This study reports about physiological and behavioral measures of simultaneous olfactory and visual information processing. In particular, emotionally loaded pictures had to be rated with respect to valence and intensity under different olfactory conditions while brain activity was recorded with magnetoencephalography (MEG). The present findings support the idea that different visually induced emotions are differently influenced by simultaneous olfactory stimulation. First, this finding underlines that different emotions are associated with different neural substrates which differently overlap with olfactory neural pathways. In terms of functional interpretations it can be inferred that odour-induced emotions differently interfere with different visually induced emotions. Without providing further evidence we would nevertheless like to mention that we believe olfaction to be the earliest emotion system per se. According to this working hypothesis, this early emotion system developed via the sense of olfaction and was then used by other sensory modalities to evaluate their respective input. Of course further investigations are needed to test this hypothesis. Reports like, odours change the pitch of voice in humans might also point into a very basic direction of olfactory influences [
15]. Recently, the idea that odour-related emotions are different compared to other known emotions was mentioned [
16]. They stated that the examination of the nature of verbal labels that describe emotional effects elicited by odours point to a structure of affective responses to odours that differs from the classical taxonomies of emotion such as posited by discrete bidimensional emotion theories. The authors suggest that the subjective affective experience or feelings induced by odours are structured around a small group of dimensions that reflect the role of olfaction in well-being, social interaction, danger prevention, arousal or relaxation sensations, and conscious recollection of emotional memories. Our study provides behavioural and physiological evidence about different emotional qualities between olfaction and vision.
4.1. Behavioural data
Behavioural data analysis revealed various significant effects related to changes in subjective ratings of valence intensity due to simultaneous olfactory stimulation. By far the most dominant behavioural influence of simultaneous olfactory stimulation occurred in the
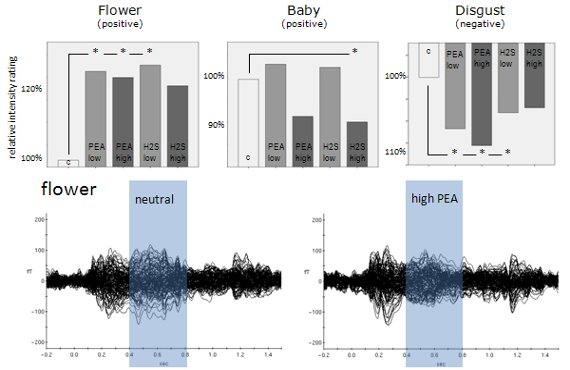
flower condition. This condition happened to be the most neutral condition in terms of visually induced emotion (see
Table 1 and
Figure 2). The mean intensity rating for
flowers in the olfactory control condition was 1.53 (positive) whereas it was 2.48 (positive) for
erotic pictures and 2.59 (positive) for
baby pictures. In the
erotic condition no significant effect occurred. In the
baby condition a slight significant reduction in emotion intensity rating occurred (92.7% of neutral) in case of high concentration negative odour. In the
flower condition we found an increase in emotion intensity rating of 126.1% in case of low concentration positive odour, 124.2% in case of high concentration positive odour and 127.5% in case of low concentration negative odour. Thus, positive valence ratings in low intensity ranges seemed to be mostly affected by simultaneous olfactory stimulation.
In contrast, for visually induced negative emotion the most dominant effect occurred in the condition which was associated with highest intensity ratings, in this case disgust. The mean valence intensity rating for the neutral odour condition for disgusting pictures was 3.6 (negative). Small but significant increases of 106.7% in negative valence intensity rating occurred in the low concentration positive odour condition, 108.6% in the high concentration positive odour condition and 104.4% in the low concentration negative odour condition. No significant effects at all were found for the fear category which was associated with a mean intensity rating in the neutral odour condition of 2.13 (negative). Therefore, negative valence ratings in high intensity ranges seemed to be mostly affected by simultaneous olfactory stimulation.
This different pattern of behaviour-related results for positive and negative visually induced emotion underlines the notion of qualitatively different systems related to these opposite valence directions. In the positive valence direction less visually induced emotion intensity was most obviously influenced by simultaneous olfaction whereas in the negative valence direction it was high visually induced emotion intensity. Interestingly, in a previous fMRI study it was found that emotion-related arousal and pleasantness related to odors are processed independently by different brain regions [
17]. More importantly the authors demonstrated that for olfaction, the amygdale had no intrinsic preference for negative emotion after the effects of arousal had been controlled. This could explain why in our study olfactory influences occurred at particular intensity levels which were different between positive and negative visually induced emotions. A further interpretation of our behavioural results is that the negative emotion of fear was not affected by simultaneous olfactory stimulation in terms of modifying intensity ratings because it heavily involved amygdale activation. As already mentioned in the introduction, the amygdale are also known for their involvement in olfactory information processing, especially at early processing stages. We can therefore infer that both sensory modalities engaged the amygdale and subjective rating of visually induced emotion occupies these neural structures more dominantly than olfaction not allowing it to interfere.
4.2. Physiological data
Physiologically, we investigated whether the different olfactory conditions elicited different patterns of effects with respect to our five picture emotion categories. After all, the analysis of brain activity data revealed different patterns of significant effects across emotion categories demonstrating different influences of simultaneous olfactory stimulation on brain processes engaged in different emotion-related information processing triggered through the sense of vision. The fact that we introduced hemisphere as a factor in our statistical analysis goes back to previous findings about lateralisation related to odour information processing. Previous work about hemispheric differences related to odour processing in the human brain has been summarised [
18]. The authors found that the left hemisphere participates more in processing emotion-related information of an olfactory stimulus than the right hemisphere. On the other hand, differential hemisphere involvement with a right-side advantage for processing on unpleasant affect in olfaction was suggested [
19]. These inconsistent findings left us with the supportive idea to include hemisphere in our statistical analysis but we did not further analyse our data with respect to lateralisation phenomena. Thus, we only report about the principal physiological finding that odours have different effects on different visually induced emotions.
Although in our study differences occurred between emotion categories there is evidence for at least one common effect in all of them at an early processing stage. Repeated measures ANOVAs including all five olfactory conditions (control, low concentration PEA, high concentration PEA, low concentration H
2S and high concentration H
2S) and separately calculated for each visual emotion category and for each individual 100 ms time window revealed common significances across emotional categories between about 250 ms and 400 ms after stimulus onset. Only in the normalized data set in the emotion category
erotic no such significances occurred. Anyway, this early time window is known to reflect brain activities engaged in subconscious olfactory information processing [
2–
4]. It can therefore be concluded that subconscious olfaction-related information processing most consistently varies across all five picture emotion categories. However, as mentioned above, the only emotion category without significant olfaction-related effects in this early time window in the normalized data set was
erotic. It has to be emphasized though that respective p-values show a strong trend towards significance, but they were above the threshold of 0.05. This is why this emotion category deserves further consideration, especially, because behavioural data analysis also revealed no significant effect in the
erotic emotion category. This coincidence points to a correlation between brain activities and behaviour, however, we cannot draw this general conclusion, because the rest of the categories does not show any sign of such a correlation. Nevertheless, the fact that neither physiological recordings related to erotic images nor subjective valence intensity ratings related to them were influenced by simultaneous olfactory stimulation is puzzling. One possible explanation for this finding is that the direct relation of erotic content to conservation of the own species is so robust that its elicited emotion is not that vulnerable to simultaneous olfaction as in other picture emotion categories. This notion is underlined by the fact that subjective valence intensity ratings related to the emotion category of
fear weren’t affected by simultaneous olfaction either. Fear is directly related to the conservation of oneself which might also reflect a rather robust process not easily influenced by emotion elicited through other sensory modalities. However, physiologically the emotion category
fear was associated with significant olfactory effects, in contrast to the emotion category
erotic.
Most obviously, different patterns of significant olfaction-related differences between picture emotion categories occurred during later time intervals (
Figure 3). Whereas the early time window around 300 ms after stimulus onset reflects subconscious olfactory information processing, later intervals around 700 ms after stimulus onset were previously found to reflect conscious odour perception [
2–
4]. We found three emotion categories to be associated with significant olfaction-related influences in this later time window,
flower,
fear and
disgust. Although varying a bit in terms of exact temporal occurrence only these emotion categories showed significant olfaction-related effects after the earlier consistent effect which was found for all emotion categories. This finding provides evidence for different conscious odour-related influences on these emotion categories only.
Figure 3 shows event-related magnetic field (ERF) distributions for the neutral olfactory condition and the high concentration PEA olfactory condition (most dominant physiological effects were found for PEA high concentration) of these three emotion categories. As can be seen, in all three emotion categories high concentration PEA stimulation resulted in reduced ERF amplitudes in later time intervals. This physiological situation corresponds with increases in intensity ratings irrespective of valence direction. However, in case of the emotion category
fear increases in intensity rating were not significant.
The emotion category
flower exhibited most pronounced later olfactory-related brain activities and it was also the
flower category which was most dominantly affected with respect to changes in valence intensity ratings. We can therefore infer that the later component reflecting conscious odour perception was mostly responsible for the significant behavioural changes. Only simultaneous conscious odour perception modifies explicit subjective ratings related to visually induced emotion. A similar conclusion was previously drawn [
3] in a study about olfaction and word processing. In that study, it was concluded that only simultaneous conscious olfaction during deep word processing caused reductions in later word recognition performance. Further evidence for olfaction and language interactions was also presented earlier [
20].
As already mentioned, separate comparisons between the chemical control condition and every single olfactory condition for each emotion category revealed the most pronounced effects for the high concentration PEA (phenylethyl alcohol) condition. At the moment we don't want to further discuss detailed effects of particular odour conditions, but it seems that further investigations focusing on this issue might lead to promising results with respect to a better understanding of emotions related to olfaction.
After all, the present study provides evidence of variable interference between olfaction and visually induced emotion depending on what emotion is visually processed and depending on its perceived intensity. Follow up studies are planned with more study participants to calculate possible gender differences. It is well known that gender plays an important role in olfaction and maybe some of the data variability leading to insignificant results goes back to gender differences. Also, better control of valence and intensity (arousal) related to picture presentations will help to better understand selective influences related to both dimensions.