Design of Selective Gas Sensors Using Additive-Loaded In2O3 Hollow Spheres Prepared by Combinatorial Hydrothermal Reactions
Abstract
: A combinatorial hydrothermal reaction has been used to prepare pure and additive (Sb, Cu, Nb, Pd, and Ni)-loaded In2O3 hollow spheres for gas sensor applications. The operation of Pd- and Cu-loaded In2O3 sensors at 371 °C leads to selective H2S detection. Selective detection of CO and NH3 was achieved by the Ni-In2O3 sensor at sensing temperatures of 371 and 440 °C, respectively. The gas responses of six different sensors to NH3, H2S, H2, CO and CH4 produced unique gas sensing patterns that can be used for the artificial recognition of these gases.1. Introduction
Chemoresistive n-type oxide semiconductors such as SnO2, ZnO, TiO2, In2O3, and WO3 have been widely used to detect explosive, toxic and harmful gases [1,2]. The main advantages of oxide semiconductor sensors are the simple and cost-effective detection of various gases. High gas responses for detecting trace concentrations of analyte gases can be accomplished by employing well-defined nanostructures [3]. However, selective gas detection using oxide semiconductor sensors is often difficult because a number of different reducing gases can interact electrochemically with negatively charged surface oxygen. Various approaches have been employed to enhance the selectivity of sensors, which include the manipulation of sensing temperature [4,5], the addition of noble metal and oxide catalysts [6,7], coating with a catalytic filtering layer [8], compositional control of composite sensing materials [9], and the use of a neural network algorithm [10].
Combinatorial chemistry provides an attractive and promising approach for high-throughput screening of medicine, catalysts, and functional materials [11–14]. Generally, the combinatorial methods usually use parallel synthesis or characterization for high-speed screening. However, combinatorial approaches can be also applied to the compositional design of composite materials. Accordingly, most of the approaches to achieve highly selective gas sensors through the compositional control of sensing materials, catalysts and additives can be best optimized using combinatorial approaches [15–17]. Moreover, abundant gas sensing characteristics attained by combinatorial investigation can be used as a valuable gas sensing library for the discrimination of complex chemical quantities via the pattern recognition mechanism. Several recent researches have verified the potential of combinatorial approaches for the development of high performance gas sensors [18–22].
Hollow structures are promising nanoarchitectures for the applications of gas sensors on account of their high surface area and gas accessible configurations of thin shells [23,24]. Not only the outer surfaces but also the inner ones participate in the gas sensing reaction. In general, oxide hollow structures are prepared by applying a coating of metal precursors onto polymeric spheres and subsequent removal of sacrificial templates by heat treatment [25,26]. Among various template-based synthetic routes, hydrothermal reaction of a solution containing a metal precursor and glucose or sucrose provides a simple, one-pot method to prepare metal-precursor-coated carbon spheres [27,28]. Hydrothermal condensation of glucose or sucrose into carbon spheres with hydrophilic surfaces [29] enables the uniform coating of metal precursors [27]. Indeed, oxide hollow structures prepared by glucose- or sucrose-mediated hydrothermal reaction showed high gas responses [28,30].
In this contribution, various metal or metal oxide additives are loaded onto In2O3 hollow spheres in a combinatorial manner by one-pot hydrothermal reaction of a solution containing glucose, In-precursors, and additive-precursors with subsequent heat treatment, and the gas responses to CH4, NH3, H2, CO, and H2S have been measured. The main focus of the study is directed at the high-throughput screening of selective gas sensors by combinatorial control of oxide additives and sensor temperatures.
2. Experimental Section
Indium (III) nitrate hydrate [In(NO3)3·xH2O, 99.9% metal basis, Sigma-Aldrich, Co.], copper (II) chloride dehydrate (CuCl2·2H2O, 99% Cica-reagent, Kanto Chem. Co.), niobium (V) pentachloride (NbCl5, 99%, Sigma-Aldrich, Co.), nickel (II) chloride hexahydrate (NiCl2·6H2O, 99.9%, Sigma-Aldrich, Co.), palladium (II) chloride (PdCl2, 99%, Sigma-Aldrich, Co.), antimony (III) chloride (SbCl3, 98%, Kanto Chem. Co.) and d-(+)-glucose monohydrate (C6H12O6·H2O, 99.5%, Sigma-Aldrich, Co.) were purchased and used without further purification.
Pure and additive-loaded In2O3 hollow spheres were prepared by glucose-mediated hydrothermal reaction. d-(+)-Glucose monohydrate (5.9451 g) was dissolved in distilled water (60 mL). Subsequently, indium (III) nitrate hydrate (0.6017 g) was dissolved and stirred for 15 min. This solution was used for the preparation of the pure In2O3 hollow spheres. For the preparation of additive-loaded In2O3 hollow spheres, the corresponding amount (1 wt% compared to In2O3) of additive source was added to the above solution. These stock solutions were transferred into a Teflon-lined stainless steel autoclave, which was then sealed and heated at 180 °C for 24 h. After hydrothermal reaction, the product was washed with distilled water 4 times and ethanol 1 time by centrifuge and dried at 70 °C for 24 h. The pure and additive-loaded In2O3 hollow spheres could be prepared by the heat treatment of the above products at 500 °C for 2 h. For simplicity, hereinafter, the pure, Cu, Nb, Ni, Pd, Sb-loaded In2O3 hollow spheres after heat treatment will be referred as In2O3, Cu-In2O3, Nb-In2O3, Pd-In2O3, Ni-In2O3, and Sb-In2O3 specimens, respectively. The morphologies of the hollow spheres were analyzed by field-emission scanning electron microscopy (FE-SEM, S-4800, Hitachi Co. Ltd.).
For the gas sensing measurement, 0.1 g of each prepared hollow sphere was dispersed in 10 mL of D.I. water and these solutions were deposited on the sensor substrate by using the drop-coating technique. An alumina substrate (1.5 × 1.5 mm2) with two Au electrodes on its top surface and a micro-heater on its bottom surface was used. The temperature of the sensors was controlled by modulating the power of the microheater underneath the substrate. The sensor temperature was measured to be 371 and 440 °C at the heater powers of 400 and 500 mW, respectively, by an IR temperature sensor (Rayomatic 14814-2, Euroton IRtec Co.). The uncertainty of sensor temperature was ±5 °C. The sensor was positioned in a specially designed quartz tube chamber and dry synthetic air and mixing gas were flowed into this chamber. The gas response (S = Ra/Rg, Ra: resistance in air, Rg: resistance in gas) to 500 ppm CH4, 100 ppm NH3, H2, CO, and 5 ppm of H2S were measured using a multimeter (Keithley K2000) which connected with a computer.
3. Results and Discussion
All the as-prepared specimens after hydrothermal reaction were spheres with a size of 5–7 μm (Figure 1). The surface morphology, the presence of nano-size particles, and the connectivity between carbon spheres were slightly different for each specimen according to the doping of additives. After heat treatment of the precursor spheres at 500 °C for 2 h, the as-prepared precursor spheres with clean surfaces (Figure 1) were converted into spheres with rough surfaces consisting of primary nanoparticles (Figure 2).
The average diameters of ∼100 In2O3, Sb-In2O3, Cu-In2O3, Nb-In2O3, Pd-In2O3 and Ni-In2O3 spheres were 2.3 ± 0.5 μm, 2.4 ± 0.7 μm, 2.2 ± 0.4 μm, 2.3 ± 0.6 μm, 2.3 ± 0.5 μm, and 2.2 ± 0.5 μm, respectively. The decrease of sphere diameters during heat treatment can be attributed to the shrinkage of spheres by the decomposition of carbon cores.
Figure 3 shows the TEM images of the specimens after heat treatment at 500 °C for 2 h. All the specimens showed bright contours in the centers of spheres, which indicated the hollow morphology. The selected area electron diffraction patterns of hollow spheres were indexed as cubic In2O3 phases. The thicknesses of shells depended on the additives, which ranged from 50 nm to 200 nm.
The In2O3, Nb-In2O3, Ni-In2O3, Pd-In2O3 and Sb-In2O3 specimens after heat treatment at 500 °C for 2 h were identified as pure cubic In2O3 phase (JCPDS# 06-0416) by X-ray diffraction (XRD) (Figure 4).
The analyses of possible second phases were difficult probably due to the detection limit of XRD. In the Cu-In2O3 specimen, a small amount of corundum-type rhombohedral phase (JCPDS# 22-0336) co-existed. The rhombohedral phase is known to be stable at high pressure [31]. However, it has been reported that a metastable rhombohedral phase can be prepared by doping with Sn4+ or Fe3+ [32,33], or by sol-gel based synthesis [34]. Considering the cubic phase of the pure In2O3 specimen, the rhombohedral structure of the Cu-In2O3 specimen might be understood as the result of Cu2+ incorporation into the lattice of In2O3, although further systematic studies are necessary to confirm this.
At the sensor temperature of 371 °C, the gas responses of the In2O3 sensor to 100 ppm NH3, 5 ppm H2S, 100 ppm H2, 100 ppm CO and 500 ppm CH4 ranged from 2.0 to 4.6 (Figure 5). All the gas responses were decreased by the loading of Nb and Sb. In contrast, the loading of Cu increased the responses to all the gases. In the Cu-In2O3 sensor, although the response to H2S (7.5) was higher than the response to the other 4 gases, it was not markedly higher than the response to NH3 (6.2). Among 5 different sensors, the loading of Pd showed the most selective detection of H2S. The H2S response of the Pd-In2O3 sensor was 8.7 while the responses to NH3, H2, CO, and CH4 were 5.1, 3.0, 3.9, and 3.2, respectively. The selectivity to a specific gas was defined as “SSG/SIG” (SSG: gas response to specific gas, SIG: gas response to interference gas). The SH2S/SIG values ranged from 1.7 to 5.7. The loading of Ni increased all the gas responses. In particular, the response to CO was enhanced to a great extent. The SCO/SIG values ranged from 1.6 to 2.2. Thus, the high response to CO (12.9) with the lower cross-responses to NH3, H2S, H2, and CH4 (5.8–8.3) demonstrates that the Ni-In2O3 sensor can be used for selective CO detection.
The selectivity of the gas sensing reaction was also influenced by the variation of sensor temperature. When the sensor temperature was increased to 440 °C, the gas responses of all the sensors tended to increase (Figure 6). In the pure In2O3 sensor, the NH3 response (10.6) was the highest whereas the response to H2S (7.2) was comparable. The loadings of Sb and Nb lead to the decrease of gas responses. The responses to H2S and NH3 of the Cu-In2O3 sensor were 12.3 and 12.6, respectively, which were higher than those to H2, CO and CH4 (4.8–7.4). The responses to H2S and NH3 of the Pd-In2O3 sensor (9.9 and 8.8) were also similar to each other. Finally, the response to NH3 of the Ni-In2O3 sensor (17.1) was significantly higher than those to other gases (3.8–10.9) at the sensor temperature of 440 °C. The SNH3/SIG values ranged from 1.6 to 4.5. The above results indicate that the selectivity of the gas sensor is influenced not only by the additives but also by the gas sensing temperature [35].
The Pd-In2O3 and Cu-In2O3 sensors showed the most selective H2S detection at 371 °C. As the sensor temperature is increased to 440 °C, discrimination between H2S and NH3 becomes difficult. In the literature, the conversion of p-type CuO into metallic CuS by interaction with H2S is regarded as a key reason for the selective H2S detection of CuO-loaded SnO2 gas sensors [36–38]. The relatively high H2S sensitivity of Cu-In2O3 can be understood from this viewpoint. Pd is a representative noble metal catalyst which is used to enhance the gas sensing characteristics of oxide semiconductors. To date, Pd has been added to SnO2, ZnO, In2O3 and TiO2 sensors to improve the sensing characteristics for C2H5OH [39], NH3 [40], LPG [41], CO [42], and H2S [43]. The diverse roles of Pd additives in the gas sensing reaction seem to relate to the physico-chemical state, loading concentration, size, and distribution of the Pd catalyst. Zhang et al. [43] reported that the loading of Pd nanoparticles on the surface of ZnO nanowires by a self-assembly reaction significantly enhanced both the gas response and selectivity to H2S. This is consistent with the present result.
Selective detections of CO and NH3 were achieved when the Ni-In2O3 sensor was operated at 371 °C or 440 °C, respectively (Figures 5 and 6). The effects of NiO loading on the gas sensing characteristics of SnO2 are not always consistent in the literature. Both enhancements [44,45] and deteriorations [46,47] of gas responses have been reported. Considering that NiO is a p-type oxide semiconductor, the positive and negative roles of NiO in the gas sensing reaction can be explained by the extension of the electron depletion layer due to the formation of a p-n junction and by the counteraction of resistance variation upon exposure to reducing gases. In the present study, the enhancement of specific gas responses to CO and NH3 should be understood in the framework of catalytic promotion of the sensing reaction and/or acid/base interaction between additives and analyte gas.
High gas response to a specific gas with a negligible cross-response is desirable for selective and quantitative gas detection. The present results do not show perfect selectivity. However, the change in the gas sensing pattern by loading of different additives and by controlling sensor temperature can be used to discriminate between the gases by using a pattern recognition algorithm. To date, several combinatorial approaches have been suggested to get different gas sensing patterns [48–52]. Aronova et al. [51] reported gas sensing patterns for chloroform, formaldehyde, and benzene using SnO2 sensors loaded with various additives such as ZnO, WO3, In2O3, Pt and Pd. Siemons et al. [52] achieved the selective detection of CO and NO2 by combinatorial loading of 7 different noble metals to La-CoTiO3 sensors.
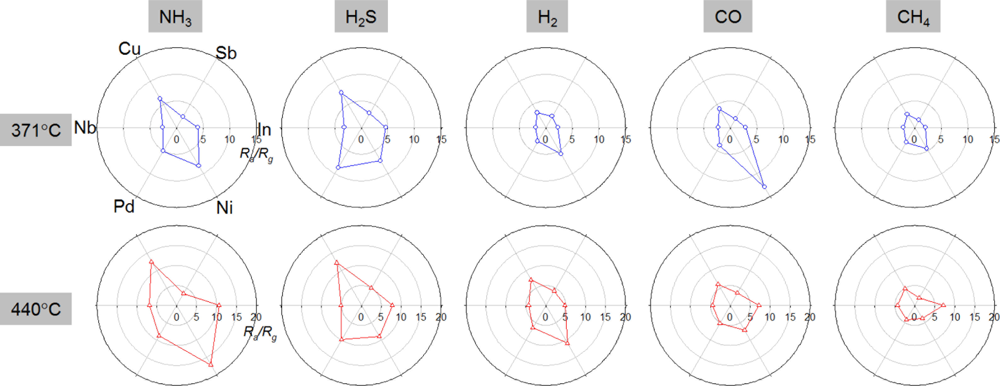
The gas sensing patterns using six different sensors were plotted in Figure 7. The relative gas responses in the polar plots showed the unique sensing patterns for each analyte gas. The operation of 6 different sensors at 371 °C can distinguish between CO and H2S. Characteristic sensing patterns of NH3, H2S, and CH4 at 440 °C facilitate gas discrimination via a pattern recognition algorithm. When this sensor array is used to detect a specific gas in real applications, the interferences from the cross-responses to other gases should be minimized by optimizing the pattern recognition algorithm considering the operation environment. The loading of various metal and metal oxide additives to In2O3 hollow spheres via the combinatorial hydrothermal route is a high-throughput approach to screening the highly selective gas sensors and to distinguishing a gas by using a pattern recognition algorithm.
4. Conclusions
Hollow spheres of pure In2O3 and Sb-, Cu-, Nb-, Pd-, and Ni-loaded In2O3 were prepared by combinatorial hydrothermal reaction of a solution containing glucose, In-precursor and additive-precursor with subsequent heat treatment. The selective detections of H2S, CO, and NH3 were achieved by the control of additives and sensing temperatures. The sensing patterns at 371 and 440 °C using six different sensors provided the characteristic signal patterns that were sufficient to discriminate between CO, H2S, NH3, H2S, and CH4. Combinatorial design of additive-loaded In2O3 hollow spheres facilitates high-throughput screening of selective gas sensors as well as the discrimination of gases via pattern recognition.
Acknowledgments
This work was supported by KOSEF NRL program grant funded by the Korean government (MEST) (No.R0A-2008-000-20032-0) and the Fundamental R&D program for Core Technology of Materials (M2008010013) funded by Ministry of Knowledge Economy.
References
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuat. B 2005, 108, 2–14. [Google Scholar]
- Shimizu, Y.; Egashira, M. Basic aspects and challenges of semiconductor gas sensors. MRS Bull 1999, 24, 18–24. [Google Scholar]
- Xu, C.N.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuat. B 1991, 3, 147–155. [Google Scholar]
- Chiorino, A.; Ghiotti, G.; Prinetto, F.; Carotta, M.C.; Gnani, D.; Marinelli, G. Preparation and characterization of SnO2 and MoOx-SnO2 nanosized powders for thick film gas sensors. Sens. Actuat. B 1999, 58, 338–349. [Google Scholar]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sen. Actuat. B 2006, 115, 610–613. [Google Scholar]
- Choi, J.-K.; Hwang, I.-S.; Kim, S.-J.; Park, J.-S.; Park, S.-S.; Jeong, U.; Kang, Y.C.; Lee, J.-H. Design of selective gas sensors using electrospun Pd-doped SnO2 hollow nanofibers. Sens. Actuat. B 2010, 150, 191–199. [Google Scholar]
- Na, C.W.; Woo, H.-S.; Kim, I.-D.; Lee, J.-H. Selective detection of NO2 and C2H5OH using a Co3O4-decorated ZnO nanowire network sensor. Chem. Commun 2011, 47, 5148–5150. [Google Scholar]
- Cabot, A.; Arbiol, J.; Cornet, A.; Morante, J.R.; Chen, F.; Liu, M. Mesoporous catalytic filters for semiconducting gas sensors. Thin Solid Films 2003, 436, 64–69. [Google Scholar]
- de Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Sivenand, P.S. Thick film organic vapour sensors based on binary mixtures. Sens. Actuat. B 2003, 92, 159–166. [Google Scholar]
- Huang, J.R.; Li, G.Y.; Huang, Z.Y.; Huang, X.J.; Liu, J.H. Temperature modulation and artificial neural network evaluation for improving the CO selectivity of SnO2 gas sensor. Sens. Actuat. B 2006, 114, 1059–1063. [Google Scholar]
- Jandeleit, B.; Schaefer, D.J.; Powers, T.S.; Turner, H.W.; Weinberg, W.H. Combinatorial materials science and catalysis. Angew. Chem. Int. Ed 1999, 38, 2494–2532. [Google Scholar]
- Koinuma, H.; Takeuchi, I. Combinatorial solid state chemistry of inorganic materials. Nature Mater 2004, 3, 429–438. [Google Scholar]
- Schultz, P.G.; Xiang, X.-D. Combinatorial approaches to materials science. Curr. Opin. Solid State Mater. Sci 1998, 3, 153–158. [Google Scholar]
- Amis, E.J. Combinatorial materials science: Reaching beyond discovery. Nature Mater 2004, 3, 83–85. [Google Scholar]
- Potyrailo, R.A.; Mirsky, V.M. Combinatorial and high-throughput development of sensing materials: The first 10 years. Chem. Rev 2008, 108, 770–813. [Google Scholar]
- Siemons, M; Simon, U. Combinatorial Methods for Chemical and Biological Sensors (Integrated Analytical Systems); Potyrailo, R.A., Mirsky, V.M., Eds.; Springer: New York, NY, USA, 2009; Chapter 11; p. 273. [Google Scholar]
- Lee, J.-H.; Kim, S.-J.; Cho, P.-S. Combinatorial Methods for Chemical and Biological Sensors (Integrated Analytical Systems); Potyrailo, R.A., Mirsky, V.M., Eds.; Springer: New York, NY, USA, 2009; Chapter 12; p. 295. [Google Scholar]
- Koplin, T.J.; Siemons, M.; Océn-Valéntin, C.; Sanders, D.; Simon, U. Workflow for high throughput screening of gas sensing materials. Sensors 2006, 6, 298–307. [Google Scholar]
- Siemons, M.; Simon, U. High throughput screening of the propylene and ethanol sensing properties of rare-earth orthoferrites and orthochromites. Sens. Actuat. B 2007, 126, 181–186. [Google Scholar]
- Siemons, M.; Koplin, T.J.; Simon, U. Advances in high throughput screening of gas sensing materials. Appl. Surf. Sci 2007, 254, 669–676. [Google Scholar]
- Kim, K.-W.; Cho, P.-S.; Kim, S.-J.; Lee, J.-H.; Kang, C.-Y.; Kim, J.-S.; Yoon, S.-J. The selective detection of C2H5OH using SnO2-ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens. Actuat. B 2007, 123, 318–324. [Google Scholar]
- Kim, S.-J.; Cho, P.-S.; Lee, J.-H.; Kang, C.-Y.; Kim, J.-S.; Yoon, S.-J. Preparation of multi-compositional gas sensing films by combinatorial solution deposition. Ceram. Int 2008, 34, 827–831. [Google Scholar]
- Lee, J.-H. Gas Sensors using Hierarchical and Hollow Oxide Nanostructures: Overview. Sens. Actuat. B 2009, 140, 319–336. [Google Scholar]
- Kim, H.-J.; Choi, K.-I.; Pan, A.; Kim, I.-D.; Kim, H.-R.; Kim, K.-M.; Na, C.W.; Cao, G.; Lee, J.-H. Template-free solvothermal synthesis of hollow hematite spheres and their applications in gas sensors and Li-ion batteries. J. Mater. Chem 2011, 21, 6549–6555. [Google Scholar]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater 2008, 20, 3987–4019. [Google Scholar]
- Caruso, F.; Shi, X.; Caruso, R.A.; Susha, A. Hollow titania spheres from layered precursor deposition on sacrificial colloidal core particles. Adv. Mater 2001, 13, 740–744. [Google Scholar]
- Titirici, M.-M.; Antonietti, M.; Thomas, A. A generalized synthesis of metal oxide hollow spheres using a hydrothermal approach. Chem. Mater 2006, 18, 3808–3812. [Google Scholar]
- Lee, C.-Y.; Kim, S.-J.; Hwang, I.-S.; Lee, J.-H. Glucose-mediated hydrothermal synthesis and gas sensing characteristics of WO3 hollow microspheres. Sens. Actuat. B 2009, 142, 236–242. [Google Scholar]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed 2004, 116, 607–611. [Google Scholar]
- Kim, S.-J.; Hwang, I.-S.; Choi, J.-K.; Kang, Y.C.; Lee, J.-H. Enhanced C2H5OH sensing characteristics of nano-porous In2O3 hollow spheres prepared by sucrose-mediated hydrothermal reaction. Sens. Actuat. B 2011, 155, 512–518. [Google Scholar]
- Shannon, R.D.; Prewitt, C.T. Synthesis and structure of a new high-pressure form of Rh2O3. J. Solid State Chem 1970, 2, 134–136. [Google Scholar]
- Yu, D.; Wang, D.; Lu, J.; Qian, Y. Preparation of corundum structure Sn-doped In2O3 nanoparticles via controlled co-precipitating and postannealing route. Inorg. Chem. Commun 2002, 5, 475–477. [Google Scholar]
- Sorescu, M.; Diamandescu, L.; Tarabasanu-Mihaila, D. α-Fe2O3–In2O3 mixed oxide nanoparticles synthesized under hydrothermal supercritical conditions. J. Phys. Chem. Solids 2004, 65, 1719–1725. [Google Scholar]
- Epifani, M.; Siciliano, P.; Gurlo, A.; Barsan, N.; Weimar, U. Ambient pressure synthesis of corundum-type In2O3. J. Am. Chem. Soc 2004, 126, 4078–4079. [Google Scholar]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sens. Actuat. B 2006, 115, 610–613. [Google Scholar]
- Tamaki, J.; Maekawa, T.; Miura, N.; Yamazoe, N. CuO–SnO2 element for highly sensitive and selective detection of H2S. Sens. Actuat. B 1992, 9, 197–203. [Google Scholar]
- Chowdhuri, A.; Sharma, P.; Gupta, V.; Sreenivas, K.; Rao, K.V. H2S sensing mechanism of SnO2 films with ultrathin CuO dotted islands. J. Appl. Phys 2002, 92, 2172–2180. [Google Scholar]
- Hwang, I.-S.; Choi, J.-K.; Kim, S.-J.; Dong, K.-Y.; Kwon, J.-H.; Ju, B.-K.; Lee, J.-H. Enhanced H2S sensing characteristics of SnO2 nanowires functionalized with CuO. Sens. Actuat. B 2009, 142, 105–110. [Google Scholar]
- Xing, L.-L.; Ma, C.-H.; Chen, Z.-H.; Chen, Y.-J.; Xue, X.-Y. High gas sensing performance of one-step-synthesized Pd-ZnO nanoflowers due to surface reaction and modifications. Nanotechnology 2011, 22, 215501. [Google Scholar]
- Zeng, Y.; Lou, Z.; Wang, L.; Zou, B.; Zhang, T.; Zheng, W.; Zou, G. Enhanced ammonia sensing performance of Pd-sensitized flowerlike ZnO nanostructure. Sens. Actuat. B 2011, 156, 395–400. [Google Scholar]
- Gurav, K.V.; Deshmukh, P.R.; Lokhande, C.D. LPG sensing properties of Pd-sensitized vertically aligned ZnO nanorods. Sens. Actuat. B 2011, 141, 365–369. [Google Scholar]
- Wei, S.; Yu, Y.; Zhou, M. CO gas sensing of Pd-doped ZnO nanofibers synthesized by electrospinning method. Mater. Lett 2010, 64, 2284–2286. [Google Scholar]
- Zhang, Y.; Xiang, Q.; Xu, J.; Xu, P.; Pan, P.; Li, F. Self-assemblies of Pd nanoparticles on the surface of single crystal ZnO nanowires for chemical sensors with enhanced performances. J. Mater. Chem 2009, 19, 4701–4706. [Google Scholar]
- Jain, K.; Pant, R.P.; Lakshmikumar, S.T. Effect of Ni doping on thick film SnO2 gas sensor. Sens. Actuat. B 2006, 113, 823–829. [Google Scholar]
- Liu, X.; Zhang, J.; Guo, X.; Wu, S.; Wang, S. Enhanced sensor response of Ni-doped SnO2 hollow spheres. Sens. Actuat. B 2011, 152, 162–167. [Google Scholar]
- Ivanovskaya, M.; Bogdanov, P. Effect of NiII ions on the properties of In2O3-based ceramic sensors. Sens. Actuat. B 1998, 53, 44–53. [Google Scholar]
- Kim, H.-R.; Choi, K.-I.; Kim, K.-M.; Kim, I.-D.; Cao, G.; Lee, J.-H. Ultra-fast responding and recovering C2H5OH sensors using SnO2 hollow spheres prepared and activated by Ni templates. Chem. Commun 2010, 46, 5061–5063. [Google Scholar]
- Scheidtmann, J.; Frantzen, A.; Frenzer, G.; Maier, W.F. A combinatorial technique for the search of solid state gas sensor materials. Nanotechnology 2006, 16, 119–127. [Google Scholar]
- Klingvall, R.; Lundström, I.; Löfdahl, M.; Eriksson, M. A combinatorial approach for field-effect gas sensor research and development. IEEE Sensors J 2005, 5, 995–1003. [Google Scholar]
- Potyrailo, R.A.; Leach, A.M. Selective gas nanosensors with multisize CdSe nanocrsystal/polymer composite films and dynamic pattern recognition. Appl. Phys. Lett 2006, 88, 134110. [Google Scholar]
- Aronova, M.A.; Chang, K.S.; Takeuchi, I.; Jabs, H.; Westerheim, D.; Gonzalez-Martin, A.; Kim, J.; Lewis, B. Combinatorial libraries of semiconductor gas sensors as inorganic electronic noses. Appl. Phys. Lett 2003, 83, 1255–1257. [Google Scholar]
- Siemons, M.; Simon, U. Preparation and gas sensing properties of nanocrystalline La-doped CoTiO3. Sens. Actuat. B 2006, 120, 110–118. [Google Scholar]






© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, S.-J.; Hwang, I.-S.; Kang, Y.C.; Lee, J.-H. Design of Selective Gas Sensors Using Additive-Loaded In2O3 Hollow Spheres Prepared by Combinatorial Hydrothermal Reactions. Sensors 2011, 11, 10603-10614. https://doi.org/10.3390/s111110603
Kim S-J, Hwang I-S, Kang YC, Lee J-H. Design of Selective Gas Sensors Using Additive-Loaded In2O3 Hollow Spheres Prepared by Combinatorial Hydrothermal Reactions. Sensors. 2011; 11(11):10603-10614. https://doi.org/10.3390/s111110603
Chicago/Turabian StyleKim, Sun-Jung, In-Sung Hwang, Yun Chan Kang, and Jong-Heun Lee. 2011. "Design of Selective Gas Sensors Using Additive-Loaded In2O3 Hollow Spheres Prepared by Combinatorial Hydrothermal Reactions" Sensors 11, no. 11: 10603-10614. https://doi.org/10.3390/s111110603



