A Renewable and Ultrasensitive Electrochemiluminescence Immunosenor Based on Magnetic RuL@SiO2-Au~RuL-Ab2 Sandwich-Type Nano-Immunocomplexes
Abstract
: An ultrasensitive and renewable electrochemiluminescence (ECL) immunosensor was developed for the detection of tumor markers by combining a newly designed trace tag and streptavidin-coated magnetic particles (SCMPs). The trace tag (RuL@SiO2-Au∼RuL-Ab2) was prepared by loading Ru(bpy)32+(RuL)-conjuged secondary antibodies (RuL-Ab2) on RuL@SiO2 (RuL-doped SiO2) doped Au (RuL@SiO2-Au). To fabricate the immunosensor, SCMPs were mixed with biotinylated AFP primary antibody (Biotin-Ab1), AFP, and RuL@SiO2-Au∼RuL-Ab2 complexes, then the resulting SCMP/Biotin-Ab1/AFP/RuL@SiO2-Au∼RuL-Ab2 (SBAR) sandwich-type immunocomplexes were absorbed on screen printed carbon electrode (SPCE) for detection. The immunocomplexes can be easily washed away from the surface of the SPCE when the magnetic field was removed, which made the immunosensor reusable. The present immunosensor showed a wide linear range of 0.05–100 ng mL−1 for detecting AFP, with a low detection limit of 0.02 ng mL−1 (defined as S/N = 3). The method takes advantage of three properties of the immunosensor: firstly, the RuL@SiO2-Au∼RuL-Ab2 composite exhibited dual amplification since SiO2 could load large amount of reporter molecules (RuL) for signal amplification. Gold particles could provide a large active surface to load more reporter molecules (RuL-Ab2). Accordingly, through the ECL response of RuL and tripropylamine (TPA), a strong ECL signal was obtained and an amplification analysis of protein interaction was achieved. Secondly, the sensor is renewable because the sandwich-type immunocomplexes can be readily absorbed or removed on the SPCE’s surface in a magnetic field. Thirdly, the SCMP modified probes can perform the rapid separation and purification of signal antibodies in a magnetic field. Thus, the present immunosensor can simultaneously realize separation, enrichment and determination. It showed potential application for the detection of AFP in human sera.1. Introduction
The clinical analysis of cancer biomarkers is critical to the early diagnosis of cancer and proteomics research, which will also promote the understanding of cancer diseases’ related biological processes [1–3]. Alpha-fetoprotein (AFP) is a well-known tumor marker related to hepatocellular carcinoma (HCC) [4]. The average concentration of AFP in serum often increases under cancer conditions from 5–20 ng mL−1 [5]. Thus the development of ultrasensitive detecting methods for trace levels of AFP in human serum is important for the early diagnosis of cancers. Compared with conventional immunoassays such as enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassay, the ECL assay not only shows high sensitivity and wide dynamic concentration response range, but also is potential and spatially controlled [6–10]. Among various ECL systems, Ru(bpy)32+-based ECL has gained importance owing to its superior properties of high sensitivity and good stability in aqueous solution under moderate conditions [11,12]. Recently, some stable and sensitive ECL biosensors based on Ru(bpy)32+ doped SiO2 nanoparticles have been intensively investigated [13–15]. On one hand, SiO2 nanoparticles are considered a good matrix because their surfaces are easily functionalized and conjugated to bioactive molecules, which shows great potential in bioanalysis. On the other hand, the SiO2 matrices can resist dye molecules coming off and this increases their photostability [13,16]. In addition, gold nanoparticles (Au NPs) have gained attention in the last years due to the unique structural, electronic, optical, and catalytic properties which have made them become very attractive materials for biosensor systems and bioassays [17]. Yuan and co-workers have proposed a novel sensitive ECL immunoassay with AFP antibody labeled RuL@SiO2-Au as probes [18]. However it required complex modification procedures on the electrode. Furthermore the sensor cannot be renewed after the immune products are formed on electrode.
Conventional electrodes, such as glassy carbon electrode (GCE), Au electrode or magnetic carbon paste electrode (MCPE), are relatively expensive and bulky. Moreover, they are not convenient to add or remove the magnetic iron on the long cylindrical electrode to attract the magnetic nanoprobes. However, amperometric biosensors fabricated with screen printed carbon electrodes (SPCEs) have the advantages of integration of electrodes, simple manipulations, low cost and low consumption of sample which can be used for one step determination, and then discarded [19,20]. Recently, magnetic nanoparticles have also gained increasing interest and have been widely applied in immunoassays [21,22] due to their biocompatibility, superparamagnetism and good electron conductivity [23], which can simplify the process of protein immobilization and separation [24]. The magnetic nanoprobes strategy developed recently has proven to be a highly sensitive technique for detecting human tumor cells, and is especially well suited to separate and at the same time detect low-concentrations of proteins [25,26]. More importantly, the magnetic probes can be modified and removed from the surface by a magnetic field added on the flat bottom of SPCEs. All these steps can make the electrode’s surface renewable and simplify the electrode modification steps. Furthermore, if a RuL labeled antibody is employed instead of pure antibody to be labeled on RuL@SiO2-Au nanoparticles as probes (RuL@SiO2-Au∼RuL-Ab2), the relevant ECL signal could greatly improve due to the increased number of reporter molecules (RuL) that are modified on the electrode surface.
Our present work is motivated by the promising applications of the SBAR sandwich-type immunocomplexes for ultrasensitive detection of AFP and renewable surface of SPCEs. In this study, the streptavidin-coated magnetic particles (SCMP), as supporting material, not only perform the rapid separation and purification of signal antibody on magnetic field, but they also enhance the fixed capacity of Biotin-Ab1 to improve the detection range. Furthermore, the SBAR sandwich-type immunocomplexes were readily immobilized on the working electrode of SPCEs by magnets, therefore, unlike traditional electrochemical immune sensors, the electrodes do not require complex modification and cleaning. In addition, the RuL@SiO2-Au∼RuL-Ab2 composite exhibited dual amplification since SiO2 could adsorb large amount of reporter molecules (RuL) and gold particles could provide large active surface to load more reporter molecules (RuL-Ab2). Thus, the SCMP/Biotin-Ab1/AFP/RuL@SiO2-Au∼RuL-Ab2 (SBAR) sandwich-type immunocomplexes were easy to control and have strong ECL signals.
2. Experimental Section
2.1. Reagents and Materials
Alpha-fetoprotein antibody (Anti-AFP, 12 mg L−1) was from Biocell Company (Zhengzhou, China). Elecsys AFP Kits were from Roche Diagnostics GmbH. Tris(2,2’-bipyridyl)ruthenium(II) chloride Hex hydrate-(Ru(bpy)3Cl2 6H2O) was from Strem Chemicals. Tripropylamine (TPA) was from Tokyo Kasei Kogyo Co, Ltd. Gold chloride (HAuCl4), BSA (96∼99%) and Triton X-100 (TX-100) were bought from Sinopharm Chemical Reagent Co.Ltd (Shanghai, China). Tetraethylorthosilicate (TEOS) and 3-aminopropyltriethoxysilane (APTES) were from Aladdin Chemistry Co. Ltd. Gold nanoparticles (Au-NPs, ∼16 nm in diameter) were prepared according to the procedures reported by Enüstün et al. [27]. Phosphate buffered solution (PBS, pH 7.4) was prepared using 0.1 M Na2HPO4, 0.1 M KH2PO4 and 0.1 M KCl. Blocking buffer solution consisted of a PBS with 3% (w/v) BSA and 0.05% (v/v) Tween 20. Washing buffer solution consisted of a PBS with 0.1 M NaCl and 0.05% (v/v) Tween 20 (PBST). All other chemicals were of analytical grade and all solutions were prepared with doubly distilled water.
2.2. Apparatus
ECL experiments were carried out using a MPI-B model electrochemiluminescence analyzer (Xi’an Remax Electronic Science & Technology Co. Ltd., Xi’an, China) with the voltage of the photomultiplier tube set at 800 V and initial potential = 0.0 V, high potential = 1.2 V, scan rate = 100 mV/s. A three-electrode system was used, which consists of a screen printed carbon working electrode (SPCE), a carbon auxiliary electrode and an Ag/AgCl reference electrode (DropSens Corporation, Spain). A H600 transmission electron microscope (Hitachi, Japan) was employed to characterize the nanoparticles.
2.3. Preparation of RuL@SiO2-Au Nanoparticles
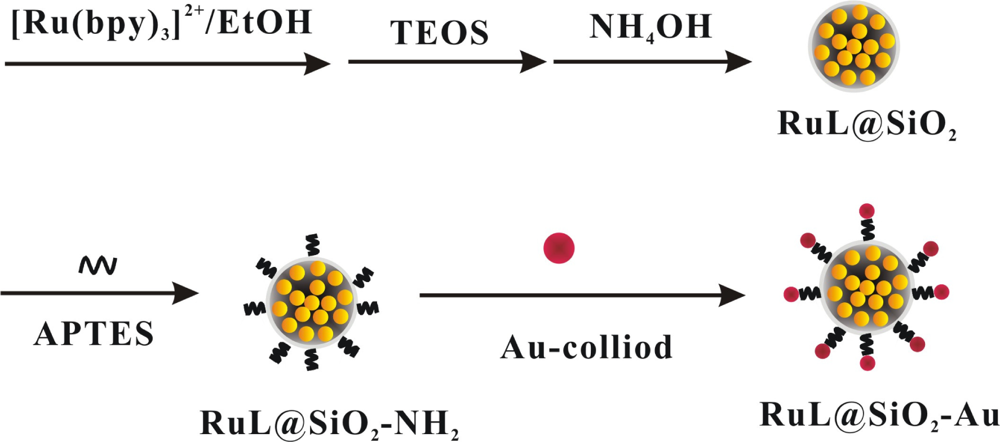
RuL@SiO2 nanoparticles were prepared according to the literature [16,28]. In brief, to a mixture of TX-100 (1.77 mL), cyclohexane (7.5 mL), n-hexanol (1.8 mL), 0.1 M Ru(bpy)32+ (80 μL), tetraethylorthosilicate (TEOS, 100 μL) and water (340 μL) was added NH4OH (60 μL, 25%). The mixture was stirred for 24 h, and the generated solid was isolated with acetone, sonicated for 10 min, followed by centrifuging and washing with ethanol and water. Then, the orange RuL@SiO2 nanoparticles were collected and dried in vacuum oven. To prepare the RuL@SiO2-Au nanoparticles, RuL@SiO2 nanoparticles suspension in ethanol (6 mL, 2 mg mL−1) was mixed with APTES (200 μL) and vigorously stirred for 30 min. The mixture was centrifuged and washed with water and ethanol to produce aminoterminated RuL@SiO2 nanoparticles. A mixture of Au-colloid (2 mL) and APTES-modified RuL@SiO2 suspension (1 mL) was then prepared and shaken for 1 h to let Au NPs be absorbed by the surface of the amino-terminated RuL@SiO2 nanoparticles. The thus generated RuL@SiO2-Au nanoparticles were collected by centrifugation, washed with water and suspended in water. The schematic graph of the fabrication process of RuL@SiO2 and RuL@SiO2-Au nanoparticles was shown in Scheme 1.
2.4. Preparation of RuL@SiO2-Au∼RuL-Ab2 and RuL@SiO2-Au∼Ab2 (RuL@SiO2-Au Composite Nanoparticles Labeled AFP Secondary Antibody)
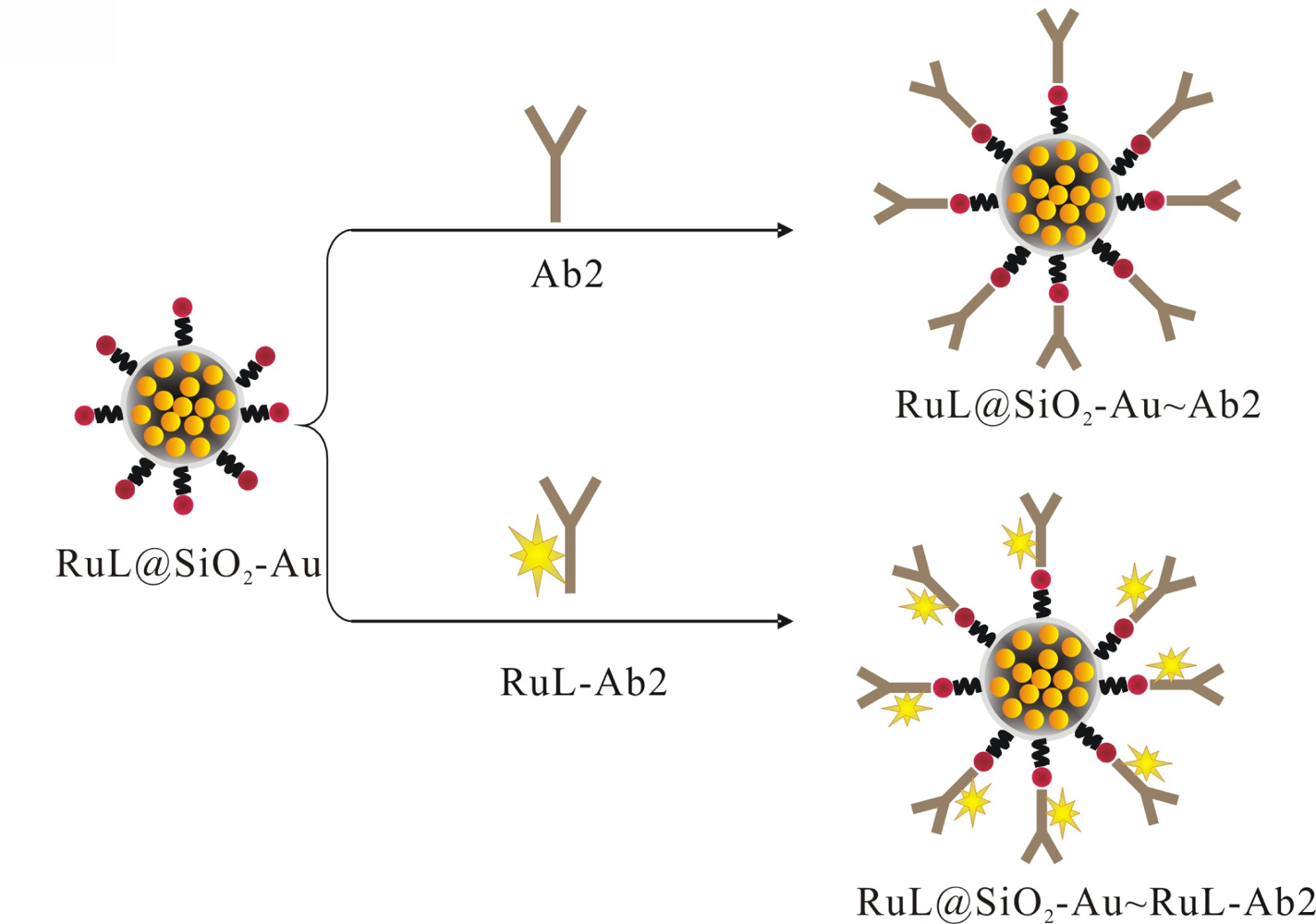
The RuL@SiO2-Au∼RuL-Ab2 bionanocomposite were prepared according to the literature [17]. In brief, a mixture of RuL@SiO2-Au nanoparticles suspension and excess RuL-Ab2 was stirred for 24 h at 4 °C. The mixture was centrifuged and washed with water to obtain the RuL@SiO2-Au∼RuL-Ab2 bionanocomposite. The as-prepared bionanocomposite was incubated with BSA to block the unreacted and nonspecific sites, and then centrifuged and washed with PBS, dispersed in 0.1 M PBS (2 mL, pH 7.4) and stored at 4 °C for further use. The RuL@SiO2-Au∼Ab2 bionanocomposite was fabricated by using the same procedure, as shown in Scheme 2.
2.5. Preparation of the SBAR Sandwich-Type Immunocomplexes
The schematic of the fabrication process is shown in Scheme 3. The immunocomplexes were prepared as follows: a mixture of Biotin-Ab1 (50 μL), different concentrations of AFP (50 μL) and RuL@SiO2-Au∼RuL-Ab2 (50 μL) was prepared and held for 10 min at room temperature. After that, streptavidin-coated magnetic particles (SMP, 50 μL) were added into the mixture, allowing the RuL@SiO2-Au∼RuL-Ab2-AFP-Ab1-Biotin sandwich type immune-complexes to be captured on the surface of magnetic particles via biotin-streptavidin interaction. Then the sandwich-type immunocomplexes were isolated by a magnet, washed with PBST solution three times, dispersed in PBS (150 μL, pH 7.4) and stored at 4 °C for ECL tests.
2.6. ECL Measurements
The three-electrode system and ECL measurement process are shown in Scheme 4. For each test, sandwich-type immunocomplex solution (5 μL) prepared with different concentrations of target AFP was attached on the cleaned SPCE surface with a NdFeB permanent magnet, ECL measurements were then performed in PBS (30 μL, pH 7.4) containing 10−5 M TPA with a photomultiplier tube voltage of 800 V.
3. Results and Discussion
3.1. Characterization of RuL@SiO2 and RuL@SiO2-Au Nanoparticles
In this work, [Ru(bpy)3]2+-doped silica matrix loaded with Au-NPs, named RuL@ SiO2-Au, was prepared as ECL signal amplification labels and immobilization substrates for AFP secondary antibody (Ab2). RuL@SiO2 nanoparticles were first fabricated by using the well-established water-in-oil (W/O) microemulsion method. Figure 1(A) shows the TEM image of RuL@SiO2 nanoparticles with a uniform size distribution (∼120 nm diameter). Incorporation of RuL molecules inside the silica matrix protects them from the surrounding environment, increases photostability and provides signal enhancement due to an increasing amount of RuL molecules doped per nanoparticle [29]. Furthermore, the ease of assembling functional groups such as amines, thiols and carboxyls on the surface of [Ru(bpy)3]2+-doped silica nanoparticles enables their use as ideal amplification labels for bioanalysis applications [30]. To immobilize AFP secondary antibody on the RuL@SiO2 matrix, the surface of RuL@SiO2 nanoparticles was aminoterminated with APTES and further reacted with Au-NPs. Figure 1(B) demonstrates that some individual Au-NPs (∼16 nm diameter) and cluster-shape Au-NPs were successfully assembled on the surface of RuL@SiO2 nanoparticles. These attached Au-NPs could provide a biocompatible, accessible matrix for immobilization of AFP secondary antibody.
3.2. Optimization of Experimental Conditions
The ECL behavior of the sandwiched immunoassay was caused by the TPA and Ru(bpy)32+. Thus TPA plays an important role in enhancement of the ECL signal. Furthermore, the enhanced ECL signal was related to the concentration of TPA. As can be seen from Figure 2, the ECL signal increased with increasing concentration of TPA. The effective enhancement occurred at the TPA concentration of 10−5 M. Thus 0.1 M of PBS containing 10−5 M TPA was selected for our measurements.
3.3. ECL Characterization of RuL@SiO2-Au∼RuL-Ab2
Prior to the use of RuL@SiO2-Au∼RuL-Ab2 as labels for the preparation of ECL immunosensors, we investigated the ECL performance of RuL@SiO2-Au∼RuL-Ab2 in the presence of TPA. For sandwich-type immunosensors, the sensitivity is mainly determined by the sensitivity of the label. In this work, the signal of the ECL immunosensor was mainly from the encapsulated RuL toward TPA oxidation. Since a large number of RuL molecules were incorporated into SiO2 nanoparticles, and due to the intrinsic good electrocatalytic activity of RuL toward TPA oxidation, we hypothesized the sensitivity of the ECL immunosensor could be greatly enhanced when RuL@SiO2-Au∼RuL-Ab2 was used as recognition element of target AFP and signal amplification label. Figure 3 shows the different modified electrodes in PBS in the presence of 10−5 M TPA. It can be seen that only a small ECL intensity change was observed at the RuL-Ab2 modified electrode (curve c). On the contrary, a remarkable ECL intensity increase was observed for the RuL@SiO2-Au∼Ab2 modified electrode (curve b) and the largest ECL intensity response occurred at the RuL@SiO2-Au∼RuL-Ab2 modified electrode (curve a). These results indicated that a large amount of RuL molecules were incorporated into the silica matrix and RuL-Ab2 could be efficiently captured on the surface of RuL@SiO2-Au nanoparticles.
3.4. Performance of the ECL Immunosensor
The highly sensitive label of the SBAR sandwich-type immunocomplexes was then used to construct ECL immunosensors for AFP detection. The double-conjugated Ru(bpy)32+ sandwich-type immunocomplexes named Biotin-Ab1/AFP/RuL@SiO2-Au∼RuL-Ab2 were formed through antigen-antibody interaction in the presence of different concentrations of AFP. They were further interacted with streptavidin-coated magnetic particles (SCMP) and attached on SCPEs by magnet for ECL measurements. Then the ECL response of RuL and TPA was recorded in 0.1 M PBS (pH 7.4) containing 10−5 M TPA.
As shown in Figure 4, EI increased with the increasing of AFP concentration ranging from 0.05 to 100 ng mL−1. A linear relation between the logarithm of EI and the logarithm of AFP concentration was obtained [Log (ΔEI) = 3.3706 + 0.4075 Log (cAFP/ng mL−1)] with a correlation coefficient R = 0.9962. The detection limit was 0.02 ng mL−1 (3σ). Such a low detection limit is better than those of previously reported AFP immunosensors. The comparison of results is shown in Table 1. The improved detection limit may be attributed to two aspects: (1) the high loading level of RuL molecules into the silica nanoparticles improved the detection limit; (2) the large amount of RuL-Ab2 absorbed onto the Au-NPs surface enhanced the access chance of the antibody-antigen interaction, especially when the AFP concentration is very low.
3.5. Specificity for the Detection of AFP
The selectivity of the immunosensor was also tested by adding possible interfering substances in the AFP-mediated sandwich-type immunoreaction. Different immunocomplexes were prepared with AFP (10 ng mL−1) or AFP (10 ng mL−1) together with the following individual interferent: carcinoembryonic antigen (CEA, 10 ng mL−1), human IgG (HIgG, 1 μg mL−1), carbohydrate antigen 19-9 (CA19-9, 10 ng mL−1), human chorionic gonadotropin antigen (HCG, 10 ng mL−1), BSA (1 μg mL−1), ascorbic acid (AA, 1 μg mL−1), dopamine (DA, 1 μg mL−1) and L-lysine (LL, 1 μg mL−1). The interference degree was evaluated by comparing the ECL intensity of a mixture of AFP and interfering substance with that of AFP alone. As can be seen from Table 1, less than 5% variation of log(EI) responding to 10 ng mL−1 AFP was observed in the presence of different interferents (Figure 5), which demonstrated a good selectivity of the developed ECL immunosensor for AFP detection.
3.6. Determination of AFP in Human Serum Samples
In order to investigate the possible application of this immunosensor in clinical analysis, recovery experiments were performed by standard addition methods in human serum. The experimental results were shown in Table 2 and the recovery was in the range from 95% to 110%, which indicated that the developed sensor might be applied for the determination of AFP in human serum for routine clinical diagnosis.
3.7. Regeneration
The magnetic probe (SBAR) can be modified and removed from its surface by magnetic field added on the flat bottom of SPCEs. All these steps can renew the electrode surface and make the electrode reusable. As Figure 6 showed cyclic voltammetry (CV) curves of different electrodes (a, bare SPCEs; b, the immunocomplexes were immobilized on the working electrode of SPCEs surface; c, after renewing of SPCEs.) in 1 mM K3Fe(CN)6 (0.1 M KCl), well-defined CVs, characteristic of diffusion-limited redox processes, are observed at the bare SPCEs [Figure 6(a)]. The peak currents decreased [Figure 6(b)] after SBAR sandwich-type immunocomplexes were immobilized on the working electrode of SPCEs surface because the immunocomplexes would block the electron transfer. After removing the magnet and rinsing the sensor with PBST, the currents was further increased [Figure 6(c)] almost black to bare SPCEs, due to the bulk of immunocomplexes were rinsed out.
4. Conclusions
In this paper, a magnetic probe (SBAR) consisting of streptavidin-coated magnetic particles (SCMP) and RuL@SiO2-Au∼RuL-Ab2 complexes is used to detect AFP as a model protein. SCMP were used as supporting material for the preparation of the sandwich-type immunocomplexes. A magnetic separation step was then used to isolate the complexes from the unbound components, considerably reducing incubation and washing times. Furthermore, the SBAR sandwich-type immunocomplexes can be modified and removed from its surface by magnetic field added on the flat bottom of SPCEs, therefore, the electrodes, unlike traditional electrochemical immunosensors, do not require complex modification and cleaning steps. In addition, the RuL@SiO2-Au∼RuL-Ab2 composite exhibited dual amplification since SiO2 could load large amount of reporter molecules (RuL) and gold particles could provide large active surface to load more reporter molecules (RuL-Ab2). Thus the immunosensor can simultaneously realize separation, enrichment and determination, with high sensitivity, which would be valuable for clinical immunoassay for AFP in human serum.
Acknowledgments
The authors appreciate the support of the National Natural Science Foundation of China (No. 20805024; 81072336;10874095) and Programs for Science and Technology Development of Zhejiang, Guangdong Province and Ningbo (No.2009D1001; 2010A030300006; Y3110478; &Y3110479, 2011A610018), and the Scientific Research Foundation of Graduate School of Ningbo University (YK2009045&XYL08004), and K.C.Wong magna fund in Ningbo University.
References
- Xiao, Z; Prieto, D; Conrads, TP; Veenstra, TD; Issaq, HJ. Proteomic patterns: Their potential for disease diagnosis. Mol. Cell. Endocrinol 2005, 230, 95–106. [Google Scholar]
- Weston, AD; Hood, L. Systems biology, proteomics, and the future of health care: Toward predictive, preventative, and personalized medicine. J. Proteome Res 2004, 3, 179–196. [Google Scholar]
- Kitano, H. Systems biology: A brief overview. Science 2002, 295, 1662–1664. [Google Scholar]
- Wright, LM; Kreikemerier, JT; Fimmel, CJ. A concise review of serum markers for hepatocellular cancer. Cancer Detect. Prev 2007, 31, 35–44. [Google Scholar]
- Chou, SF; Hsu, WL; Hwang, JM; Chen, CY. Determination of [alpha]-fetoprotein in human serum by a quartz crystal microbalance-based immunosensor. Clin. Chem 2002, 48, 913–918. [Google Scholar]
- Richter, MM. Electrochemiluminescence (ECL). Chem. Rev 2004, 104, 3003–3036. [Google Scholar]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev 2008, 108, 2506–2553. [Google Scholar]
- Deng, L; Zhang, L; Shang, L; Guo, S; Wen, D; Wang, F; Dong, S. Electrochemiluminescence detection of NADH and ethanol based on partial sulfonation of sol-gel network with gold nanoparticles. Biosens. Bioelectron 2009, 24, 2273–2276. [Google Scholar]
- Choi, HN; Yoon, SH; Lyu, YK; Lee, WY. Electrogenerated chemiluminescence ethanol biosensor based on carbon nanotube-titania-nafion composite film. Electroanalysis 2007, 19, 459–465. [Google Scholar]
- Jameison, F; Sanchez, RI; Dong, L; Leland, JK; Yost, D; Martin, MT. Electrochemiluminescence-based quantitation of classical clinical chemistry analytes. Anal. Chem 1996, 68, 1298–1302. [Google Scholar]
- Honda, K; Yoshimura, M; Rao, TN; Fujishima, AJ. Electrogenerated chemiluminescence of the ruthenium Tris(2,2)bipyridyl/amines system on a boron-doped diamond electrode. Phys. Chem. B 2003, 107, 1653–1663. [Google Scholar]
- Obeng, YS; Bard, AJ. Electrogenerated chemiluminescence. 53. Electrochemistry and emission from adsorbed monolayers of a Tris(bipyridyl)ruthenium(II)-based surfactant on gold and tin oxide electrodes. Langmiur 1991, 71, 195–201. [Google Scholar]
- Zhang, LH; Dong, SJ. Electrogenerated chemiluminescence sensing platform using Ru(bpy)32+ doped silica nanoparticles and carbon nanotubes. Electrochem. Commun 2006, 8, 1687–1691. [Google Scholar]
- Qian, J; Zhou, ZX; Cao, XD; Liu, SQ. Electrochemiluminescence immunosensor for ultrasensitive detection of biomarker using Ru(bpy)32+-encapsulated silica nanosphere labels. Anal. Chim. Acta 2010, 665, 32–38. [Google Scholar]
- Yang, X; Yuan, R; Chai, YQ; Zhuo, Y; Mao, L; Yuan, SR. Ru(bpy)32+-doped silica nanoparticles labeling for a sandwich-type electrochemiluminescence immunosensor. Biosens. Bioelectron 2010, 25, 1851–1855. [Google Scholar]
- Zhang, LH; Dong, SJ. electrogenerated chemiluminescence sensors using Ru(bpy)32+ doped in silica nanoparticles. Anal. Chem 2006, 78, 5119–5123. [Google Scholar]
- Zhu, AW; Tian, Y; Liu, HQ; Luo, YP. Nanoporous gold film encapsulating cytochrome c for the fabrication of a H2O2 biosensor. Biomaterials 2009, 30, 3183–3188. [Google Scholar]
- Yuan, SR; Yuan, R; Chai, YQ; Mao, L; Yang, X; Yuan, YL; Niu, H. Sandwich-type electrochemiluminescence immunosensor based on Ru-silica@Au composite nanoparticles labeled anti AFP. Talanta 2010, 82, 1468–1471. [Google Scholar]
- Albuquerque, YDTD; Ferreira, LF. Amperometric biosensing of carbamate and organophosphate pesticides utilizing screen-printed tyrosinase-modified electrodes. Anal. Chim. Acta 2007, 596, 210–221. [Google Scholar]
- Nunes, GS; Jeanty, G; Marty, JL. Enzyme immobilization procedures on screen-printed electrodes used for the detection of anticholinesterase pesticides Comparative study. Anal. Chim. Acta 2004, 523, 107–115. [Google Scholar]
- Ho, K; Tsai, P; Lin, Y; Chen, Y. Using biofunctionalized nanoparticles to probe pathogeni bacteria. Anal. Chem 2004, 76, 7162–7168. [Google Scholar]
- Mikhaylova, M; Kim, DK; Berry, CC; Zagorodni, A; Toprak, M; Gurtis, ASG; Muhammed, M. BSA immobilization on amine-functionalized superparamagnetic iron oxide nanoparticles. Chem. Mater 2004, 16, 2344–2354. [Google Scholar]
- Lee, J; Lee, D; Oh, E; Kim, J; Kim, YP; Jin, S; Kim, HS; Hwang, Y; Kwak, JH; Park, JG; et al. Preparation of a magnetically switchable bio-electrocatalytic system employing cross-linked enzyme aggregates in magnetic mesocellular carbon foam. Angew. Chem. Int. Ed. Engl 2005, 44, 7427–7432. [Google Scholar]
- Perez, JM; Simeone, FJ; Tsourkas, A; Josephson, L; Weissleder, R. Peroxidase substrate nanosensors for MR imaging. Nano Lett 2004, 4, 119–122. [Google Scholar]
- Zhang, J; Song, S; Zhang, L; Wang, L; Wu, H; Pan, D; Fan, C. Sequence-specific detection of femtomolar DNA via a chronocoulometric DNA sensor (CDS): Effects of nanoparticle-mediated amplification and nanoscale control of DNA assembly at electrodes. J. Am. Chem. Soc 2006, 128, 8575–8580. [Google Scholar]
- Ambrosi, A; Castaneda, MT; Killard, AJ; Smyth, MR; Alegret, S; Merkoci, A. Double-codified gold nanolabels for enhanced immunoanalysis. Anal. Chem 2007, 79, 5232–5240. [Google Scholar]
- Enüstün, BV; Turkevich, J. Coagulation of colloidal gold. J. Am. Chem. Soc 1963, 85, 3317–3328. [Google Scholar]
- Stöber, W; Fink, A; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci 1968, 26, 62–69. [Google Scholar]
- Bagwe, RP; Yang, CY; Hilliard, LR; Tan, WH. Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 2004, 20, 8336–8342. [Google Scholar]
- Wang, L; Yang, CY; Tan, WH. Dual-luminophore-doped silica nanoparticles for multiplexed signaling. Nano Lett 2005, 5, 37–43. [Google Scholar]
- Liu, X; Wu, H; Zheng, Y; Wu, Z; Jiang, J; Shen, G; Yu, R. A sensitive electrochemical immunosensor for a-fetoprotein detection with colloidal gold-based dentritical enzyme complex amplification. Electroanalysis 2010, 22, 244–250. [Google Scholar]
- Ding, CF; Zhao, F; Ren, R; Lin, JM. An electrochemical biosensor for alpha-fetoprotein based on carbon paste electrode constructed of room temperature ionic liquid and gold nanoparticles. Talanta 2009, 78, 1148–1154. [Google Scholar]
- Zhang, SS; Zou, J; Yu, FL. Investigation of voltammetric enzyme-linked immunoassay based on a new system of HAP-H2O2-HRP. Talanta 2008, 76, 122–177. [Google Scholar]
- Huang, H; Zheng, XL; Zheng, JS; Pan, J; Pu, XY. Rapid analysis of alpha-fetoprotein by chemiluminescence microfluidic immunoassay system based on super-paramagnetic microbeads. Biomed. Microdevices 2009, 11, 213–216. [Google Scholar]
- Wang, GL; Xu, JJ; Chen, HY; Fu, SZ. Label-free photoelectrochemical immunoassay for α-fetoprotein detection based on TiO2/CdS hybrid. Biosens. Bioelectron 2009, 25, 791–796. [Google Scholar]








| Assay method | Linear range (ng mL−1) | LOD (ng mL−1) | Detection antibody | Reference |
|---|---|---|---|---|
| Electrochemistry assay | 1.00–500.0 | 0.8 | Au-Alkaline phosphatase-labeled antibody | [31] |
| 0.50–80.0 | 0.25 | HRP-antibody | [32] | |
| Voltammetric ELISA | 0.10–200 | 0.10 | HRP-antibody | [33] |
| Chemiluminescence | 1.0–800 | 0.23 | HRP-antibody | [34] |
| Photoelectrochemistry | 0.05–50 | 0.04 | Label-free | [35] |
| Electrochemiluninescence | 0.05–50 | 0.03 | Ru-silica@Au-antibody | [17] |
| 0.05–100 | 0.02 | RuL@SiO2-Au-RuL-antibody | This work |
| Sample number | Added (/ng mL−1) | Found (/ng mL−1) | Recovery (/%) |
|---|---|---|---|
| 1 | 0.50 | 0.51 ± 0.02 | 102.0 |
| 2 | 1.00 | 0.97 ± 0.02 | 97.0 |
| 3 | 5.00 | 5.28 ± 0.2 | 105.6 |
| 4 | 10.0 | 9.8 ± 0.3 | 98.0 |
| 5 | 50.0 | 47.6 ± 2.3 | 95.2 |
1Mean value ± SD of three measurements.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gan, N.; Hou, J.; Hu, F.; Cao, Y.; Li, T.; Guo, Z.; Wang, J. A Renewable and Ultrasensitive Electrochemiluminescence Immunosenor Based on Magnetic RuL@SiO2-Au~RuL-Ab2 Sandwich-Type Nano-Immunocomplexes. Sensors 2011, 11, 7749-7762. https://doi.org/10.3390/s110807749
Gan N, Hou J, Hu F, Cao Y, Li T, Guo Z, Wang J. A Renewable and Ultrasensitive Electrochemiluminescence Immunosenor Based on Magnetic RuL@SiO2-Au~RuL-Ab2 Sandwich-Type Nano-Immunocomplexes. Sensors. 2011; 11(8):7749-7762. https://doi.org/10.3390/s110807749
Chicago/Turabian StyleGan, Ning, Jianguo Hou, Futao Hu, Yuting Cao, Tianhua Li, Zhiyong Guo, and Jun Wang. 2011. "A Renewable and Ultrasensitive Electrochemiluminescence Immunosenor Based on Magnetic RuL@SiO2-Au~RuL-Ab2 Sandwich-Type Nano-Immunocomplexes" Sensors 11, no. 8: 7749-7762. https://doi.org/10.3390/s110807749




