LuxCDABE—Transformed Constitutively Bioluminescent Escherichia coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio fischeri
Abstract
: We show that in vitro toxicity assay based on inhibition of the bioluminescence of recombinant Escherichia coli encoding thermostable luciferase from Photorhabdus luminescens is a versatile alternative to Vibrio fischeri Microtox™ test. Performance of two luxCDABE-transformed E. coli MC1061 constructs (pDNlux) and (pSLlux) otherwise identical, but having 100-fold different background luminescence was compared with the performance of V. fischeri. The microplate luminometer and a kinetic Flash-Assay test format was used that differently from Microtox test is also applicable for high throughput analysis. Toxic effects (30-s till 30-min EC50) of four heavy metals (Zn, Cd, Hg, Cu) and three organic chemicals (aniline, 3,5-dichloroaniline and 3,5-dichlorophenol) were studied. Both E. coli strains had comparable sensitivity and the respective 30-min EC50 values highly correlated (log-log R2 = 0.99; p < 0.01) showing that the sensitivity of the recombinant bacteria towards chemicals analyzed did not depend on the bioluminescence level of the recombinant cells. The most toxic chemical for all used bacterial strains (E. coli, V. fischeri) was mercury whereas the lowest EC50 values for Hg (0.04–0.05 mg/L) and highest EC50 values for aniline (1,300–1,700 mg/L) were observed for E. coli strains. Despite of that, toxicity results obtained with both E. coli strains (pSLlux and pDNlux) significantly correlated with V. fischeri results (log-log R2 = 0.70/0.75; p < 0.05/0.01). The use of amino acids (0.25%) and glucose (0.05%)-supplemented M9 medium instead of leucine-supplemented saline significantly (p < 0.05) reduced the apparent toxicity of heavy metals to both E. coli strains up to three orders of magnitude, but had little or no complexing effect on organic compounds. Thus, P. luminescens luxCDABE-transformed E. coli strains can be successfully used for the acute toxicity screening of various types of organic chemicals and heavy metals and can replace V. fischeri in certain cases where the thermostability of luciferase >30 °C is crucial. The kinetic Flash Assay test format of the bioluminescence inhibition assay facilitates high throughput analysis. The assay medium, especially in case of testing heavy metals should be a compromise: optimal for the viability/luminescence of the recombinant test strain and of minimum complexing potential.1. Introduction
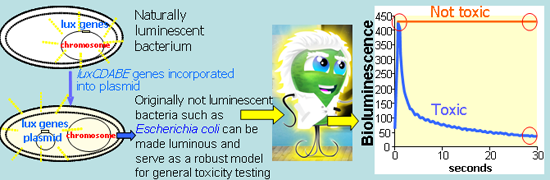
Over the last twenty five years, alternative, non-animal test systems (mainly eukaryotic cell cultures) have been introduced to supplement and, in some cases, to replace toxicity tests using animals [1], contributing to the 3R’s concept (Replacement, Reduction, Refinement of test animals) [2]. For initial toxicity screening of chemicals, bacteria are an additional attractive alternative to eukaryotic organisms. The most well-known bacterial in vitro test is Ames assay with Salmonella typhimurium [3], which may predict genotoxic effects of chemicals also to higher organisms (e.g., humans). One of the most widely used bacterial in vitro assays is also the Microtox™ test, which uses the inhibition of bioluminescence of Vibrio fischeri NRRL-B-11177 as a toxicity endpoint [4]. V. fischeri are naturally luminescent Gram-negative marine bacteria also known as Photobacterium phosphoreum NRRL-B-11177 [5] and/or Aliivibrio fischeri [6] in which luxCDABE genes are responsible for their bioluminescent reaction. When strongly expressed, a single bacterium may emit 104 or 105 photons s−1 [7]. LuxCDE genes encode a fatty acid reductase complex involved in synthesis of the long chain aliphatic aldehyde (RCHO) substrate for the luminescence reaction catalyzed by the luciferase LuxAB subunits [8]. Bacterial luciferase enzymes mediate the oxidation of reduced flavin mononucleotide (FMNH2) and RCHO by molecular oxygen (O2) to produce bioluminescence (blue-green light emission) with a maximum intensity at about 490 nm. The overall reaction can be summarized as:
For the regeneration of FMNH2 cellular NADH2 is needed and due to that the bioluminescence of the bacteria is intrinsically tied to their central metabolism. Thus, any damage of cellular metabolism caused by the toxicity of a sample could be monitored by measuring the change of light output of bacteria, the degree of toxicity being proportional to the light loss [4,9]. The V. fischeri bioluminescence inhibition assay has proven as a rapid, simple and sensitive method in toxicity testing of a wide spectrum of chemicals [10–13] and environmental samples including wastewater, solid waste, soil and sludge extracts [14–17]. A number of comparisons of the V. fischeri test (Microtox™) with different ecotoxicological assays [18] but also with various other types of toxicity assays has been made. Thus, analysis of the toxicity data for 47 MEIC reference chemicals showed that the EC50 values of P. phosphoreum bioluminescence inhibition assay correlated with literature data on acute toxicity data for daphnids, fish, animal and human cell lines, rodents, dog and man whereas the log-log correlation coefficients (R2) ranged between 0.20–0.79, depending on the data compared [19]. Thus, the naturally luminescent bacteria have already proven their potential in toxicity testing. Kinetic format of the V. fischeri test—a Flash-Assay—has been recently standardized [20] and is applicable for the analysis of sediments, solid wastes, colored samples [21] as well as for synthetic nanoparticles [13].
However, the use of V. fischeri in toxicity testing has also some limitations: due to the marine origin of bacteria, its application for freshwater and terrestrial toxicity analysis, but also for in vitro toxicity screening may raise questions. The temperature suggested for Microtox assay (+15 °C) is not compatible with conventional plate luminometers as well as needed high salinity (2% NaCl) is not suitable for mimicking certain environments. Also, for the creation of recombinant luminous constructs with temperature optimum at >30 °C, V. fischeri luciferase is not suitable due to lack of thermostability at these temperatures [22]. Due to that, luciferase encoded from luxCDABE genes from Photorhabdus luminescens has been often used for creation of recombinant luminescent bioreporters [22–25]. P. luminescens was isolated from a human wound and had the optimum temperature for growth and bioluminescence at 33 °C whereas the optimal temperature for the activity of purified luciferase was 40 °C [26]. Theoretically, the recombinant bacteria encoding thermostable luxCDABE could be a good alternative (broad temperature range, robustness) to conventional V. fischeri in toxicity screening applications. Prior to that, the performance of new recombinant bacteria with thermostable luxCDABE genes should be carefully evaluated in comparison to V. fischeri. Once validated, this test system could be also relevant for the discovering of group-, genus- or strain-specific antibacterial compounds. Some such systems have been developed and applied for (i) non-invasive in vivo bioluminescence imaging of virulent Staphylococcus aureus [27], Mycobacterium tuberculosis and M. smegmatis [28] and (ii) for studying antimicrobial efficiency of cationic peptides [29] and rhamnolipid-surfactants [30] against Pseudomonas aeruginosa.
For the further developing of such kind of models for the toxicity analysis, it is important to be sure that the sensitivity of bacteria to chemicals is attributed to the host bacterium and the test conditions and is not affected by the luminescent system used. Indeed, the bioluminescence is metabolically costly being responsible for 12% (Vibrio harveyi) and 20% (V. fischeri) of the total cellular energy requirement [31], which may theoretically compromise cellular resistance mechanisms and lead to the differences in sensitivity in bacteria with different background luminescence.
The main aim of the current study was to demonstrate that thermostable luxCDABE-transformed constitutively luminescent E. coli can be used as an alternative to V. fischeri for evaluation of acute toxicity of chemicals. Constitutively luminescent E. coli could be a suitable model for hygienic and medical microbiology for the discovering of the most effective substance against the virulent E. coli strains. We compared the toxicities of four heavy metals (Zn, Cu, Cd, Hg) and three organic chemicals (aniline, 3,5-dichloroaniline and 3,5-dichlorophenol) to two artificially bioluminescent E. coli MC1061 strains with 100-fold different background bioluminescence and naturally luminous V. fischeri. All the chosen test chemicals are important environmental toxicants and often used as biocides but differ in their modes of toxic action. To optimize the test conditions, two test media of different composition and complexing potential and exposure times of 30-s, 15-min and 30-min were used. Flash-Assay format of the bioluminescence inhibition assay in the microplate luminometer was used throughout.
2. Materials and Methods
2.1. Chemicals
All standard chemicals used were >99% of purity: ZnSO4·7H2O and CuSO4 were purchased from Alfa-Aesar (Karlsruhe, Germany); HgCl2, CdCl2·H2O and 3,5-dichlorophenol from Riedel-de-Haën Seelze, Germany). Aniline and 3,5-dichloroaniline were purchased from Sigma-Aldrich (Steinheim, Germany). The following stock solutions of standard chemicals: 27.2 mg/mL for HgCl2, 287.0 mg/mL for ZnSO4·7H2O, 159.5 mg/mL for CuSO4, 201 mg/mL for CdCl2·H2O, 8.0 mg/mL for aniline, 2.6 mg/mL for 3,5-dichlorophenol, 0.2 mg/mL for dichloroaniline were prepared in MilliQ water and stored in the dark. Before testing, the stock-solutions were diluted in MilliQ water (for E. coli tests) or in 2% NaCl (for V. fischeri tests).
2.2. Luminescent Bacterial Strains and their Preparation for Toxicity Testing
2.2.1. Escherichia coli Strains
Constitutively bioluminescent E. coli MC1061(pDNlux) and E. coli MC1061(pSLlux) constructed earlier by Ivask et al. [25] (Table 1) were used for toxicity testing. Bacteria were maintained in LB agar medium (LabM, Lancashire, UK) [32] supplemented with appropriate antibiotics (Table 1). For the toxicity tests, bacteria were cultivated (on a shaker at 200 rpm, 30 °C) overnight in 3 mL of M9 medium containing (per l): 6 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl, 0.25 g of MgSO4·7H2O, 0.01 g of CaCl2 supplemented with glucose (Cerastar, Denmark) (final concentration 0.1%), acid hydrolysate of casein (cas-AA; LabM, Lancashire, UK) (final concentration 0.5%) and appropriate antibiotics (Table 1). 1 mL of overnight culture of E. coli was added to 50 mL of fresh medium and grown till logarithmic growth phase OD600 = 0.6.
The mid-exponential phase culture was further: (i) diluted until OD600 of 0.1 with M9 medium supplemented with 0.1% glucose and 0.5% cas-AA (further referred to as “GAA-M9”) or (ii) centrifuged at 5,000 rpm for 5 min and washed twice with 0.9% NaCl supplemented with 0.01% leucine (Sigma-Aldrich, Steinheim, Germany) (further referred to as “Leu-saline”) and then diluted with the same medium till OD600 of 0.1. As in the toxicity test bacterial suspension is added to equal volume of chemical dilution prepared in water, the final composition of test media for E. coli is as follows: GAA-M9 (0.05% glucose, 0.25% casAA, 50% M9) and Leu-saline (0.005% leucine and 0.45% NaCl). Leucine is added due to the auxotrophy of this E. coli strain to leucine (Table 1) and 0.45% NaCl was optimal for luminescence of recombinant Escherichia coli strain K12 TG1, carrying luxCDABE genes of Photobacterium leiognathi 54D10 as shown by Deryabin and Aleshina [35]. For V. fischeri (Microtox™ standard operational procedure) as well as for P. phosphoreum KCTC 2852 [36] the optimal is 2% NaCl which was used also in the current study. Bacterial suspensions prepared in GAA-M9 were used for toxicity test immediately but bacterial suspensions in Leu-saline were incubated for 1.5 h at room temperature to stabilize the luminescence. The number of bacterial cells in the test was determined by counting the number of bacterial colony forming units (CFU) before the test on agarized LB media supplemented with appropriate antibiotics (Table 1). The number of E. coli cells in the test was 3.2 ± 0.63 × 107 per mL.
2.2.2. Vibrio fischeri Strains
Vibrio fischeri NRRL-B-11177 was rehydrated from Vibrio fischeri Reagent (Aboatox, Turku, Finland) using 2% NaCl (further referred to as 2% saline), stabilized for 1 h and used in test according to the procedures worked out by the manufacturers (BioTox™; Aboatox OY, Turku, Finland). The number of V. fischeri cells in the test was 1.6 ± 0.17 × 107 viable cells per mL. Testing was performed 20 °C instead of 15 °C recommended by standard operational procedure of Microtox™ (AZUR Environmental, Carlsbad, CA, USA) because very few luminometers allow to adjust the temperature at lower than 20 °C level. 20 °C has been also previously used for V. fischeri assay [37] and for Photobacterium phosphoreum KCTC 2852 strain the optimal temperature (10–30 °C were compared) for bioluminescence was 20 °C [36].
2.3. Bioluminescence Inhibition Toxicity Assay: Testing Procedure
The kinetic bioluminescence inhibition assay (Flash-Assay) was conducted essentially as described in Mortimer et al. [13]. The testing with E. coli was performed at 30 °C and with V. fischeri at 20 °C. Briefly, 100 μL of the exponentially diluted test compound(s) was pipetted into 96-well polypropylene white microplates (Greiner Bio One, Germany) in duplicate. For each chemical, 5–7 sequential exponential dilutions were analysed. Into the control wells 100 μL of MilliQ water (for E. coli) or 2% NaCl (for V. fischeri) was pipetted in four replicates instead of test chemicals. 100 μL of the bacterial suspension (prepared as described above) was automatically dispensed into the wells and after that luminescence was continuously recorded during the first 30 seconds of exposure (every 0.2 s) and then once after 15 and 30 min of incubation. Fluoroskan Ascent FL plate luminometer and the Ascent Software Version 2.4.1 (both Labsystems, Helsinki, Finland) were used to guide the dispensing and measurement. Results of 2–3 independent repeats of each experiment performed in different days were used for the calculation of EC50 values (see below). Inhibition of the luminescence (INH%) by a certain concentration/dilution of chemical was calculated as follows:
ITt—luminescence of bacteria exposed to certain concentration of chemicals after certain time of exposure (t = 30 s, 15 min, 30 min);
ICt—luminescence of bacteria in the control solution after certain time of incubation (t = 30 s, 15 min, 30 min).
30-s, 15-min and 30-min EC50 values (the concentration of chemical which reduces the luminescence of bacteria by 50% after contact time of 30-s, 15-min or 30-min, respectively) were determined from concentration versus INH% curves. The concentration-effect curves used for the 30-s, 15-min and 30-min EC50 calculations were fitted with REGTOX software for Microsoft Excel™ using the log-normal model [38]. Toxicity data for different bacterial strains and/or test conditions were compared by using t-test: two-sample assuming equal variances at p < 0.05. Statistical significance of the R values of the linear regression depending on the number of data pairs was evaluated at p = 0.10, p = 0.05 and p = 0.01.
3. Results and Discussion
Four heavy metals and three organic compounds with different mechanisms of action were chosen to compare the performance of constitutively luminescent E. coli strains and against that of V. fischeri. As previously reported, the toxic mechanism of metals is mostly due to the interference with cofactor-metals in the active sites of enzymes [39]. All the three chosen organic chemicals are according to the Verhaar classification scheme [40] categorized as polar narcotic compounds, i.e., acting non-specifically on cellular membranes. In addition, 3,5-dichlorophenol is widely used as a standard in various toxicological tests [41] and anilines are also standard chemicals in the FP6 project OSIRIS that aims to work out integrated test strategies for toxicity testing relevant for REACH [42].
3.1. The Effect of Exposure Time on the Toxicity of Chemicals to Luminescent Bacteria
For the analysis of the effect of incubation/exposure time on the test results, the toxicity of CuSO4, 3,5-dichloroaniline and aniline on E. coli MC1061(pSLlux) in Leu-saline and V. fischeri in 2% saline after 30 s, 15 and 30 min of exposure was compared. As E. coli and V. fischeri are both Gram-negative bacteria with analogous cell envelope structure, their response to most of the chemicals should be theoretically comparable. Indeed, the kinetic toxicity patterns for E. coli and V. fischeri were largely similar: both anilines but not copper reduced bacterial luminescence already after 30 s of contact. The increased toxicity of copper at 15 and 30 min of incubation compared to 30 s of exposure was significantly different (p < 0.05) for both, E. coli and V. fischeri (Figure 1). In case of V. fischeri, the increase of the toxicity of heavy metals with increasing exposure times has been shown since long time [43–45] and 30 min exposure time has been recommended for the toxicity analysis of heavy metals with Microtox assay [46]. Figure 1 shows that the kinetics of the toxic effect of anilines for E. coli was different from V. fischeri. Namely, compared with initial inhibitory effect of anilines during first 30 s of exposure their toxicity towards V. fischeri remarkably (3–4 fold) decreased at 15 or 30 min of incubation (p < 0.05) and analogous “recovery” effect was not observed for E. coli. Thus, it could be assumed that V. fischeri was rapidly adapted to the (sub)toxic effect of anilines. One of the known rapid adaption mechanisms that protect the cells against the presence of toxic organic compounds in some vibrios is the fatty acid cis-trans isomerization reaction [46,47]. This short-term in situ mechanism is in terms of metabolic energy most efficient as it is post-synthesis modification process where existing lipids are used as the substrate and de novo lipid synthesis is not required. Given the time scale of changes in toxicity that we observed in our experiments (up to 30 min), in situ cis-trans isomerization of the membrane fatty acids might be the mechanism for the recovery.
3.2. The Effect of the Test Medium Composition on the Toxicity of Chemicals to Luminescent Bacteria
The composition of the test medium to be used for acute bioluminescence inhibition assay should be a compromise: on one hand to support the stable luminescence of the bacteria during the exposure time at sufficient level needed for the detection and on the other hand, to be as little complexing as possible. The latter is especially crucial if heavy metals are analyzed [36]. However, given luminous strains of e.g., Bacillus, Staphylococcus, Pseudomonas or Escherichia are also of medical and hygienic importance, i.e., also bacterial growth inhibition data need to be studied, various nutritional supplements in the test media may be essential, depending on the auxotrophy of the strains tested. The conventional laboratory mineral growth medium for E. coli is M9 [32] that may need supplementation by various nutrients, depending on the strain. However, M9 basal medium contains phosphates and calcium (see Material and Methods) that may complex heavy metals and decrease their bioavailability to test bacteria. In addition, amino acids that must be supplemented to the growth/test media in case of amino acid auxotrophic bacterial strains may be responsible for additional complexation of heavy metals.
To study the effect of phosphate- and amino acids containing media on results of bacterial toxicity test, we compared the toxicity of seven standard chemicals to bioluminescent E. coli strains: (i) in 50% GAA-M9 medium as this medium has been used previously for these E. coli strains [25] and that in theory can also be used for chronic toxicity test and (ii) in 50% Leu-saline medium that is mimicking the test environment of the Microtox assay. Comparison of 30-min EC50 values of E. coli strains in these two media and the respective EC50 values of V. fischeri in 2% saline is presented in Table 2. For the given bacterial strains, the studied seven chemicals were of very different toxicity (the EC50 values were spanning for 4–5 orders of magnitude). However, the most toxic chemical for all bacterial strains was mercury and less toxic aniline (Table 2).
The apparent toxicity of heavy metals to E. coli in Leu-saline medium was significantly higher (p < 0.05) than in GAA-M9 medium. The biggest difference in toxicity was observed for zinc, which was more than three orders of magnitude less toxic if tested in GAA-M9. Over two orders of magnitude of decrease in toxicity were observed for cadmium (178–311 folds) and about 10–70 folds decrease for copper and mercury. Analogous test-medium dependent results were obtained for heavy metals Cu2+, Zn2+, Cd2+, and Pb2+ by Gellert et al. [48] who showed that the sensitivity of V. fischeri growth inhibition test was weakened by the presence of nutrient broth masking the toxicity by complexation. Analogously, in case of recombinant E. coli heavy metal sensor strains the reduced sensitivity of the biosensor in LB medium compared to GGM (glucose-glycerophosphate minimal medium) was observed, probably due to metal chelation or precipitation by LB medium components [49]. In case of anilines their toxicity to E. coli in both analysis media was not differing (p > 0.05) but 3,5-dichlorophenol in GAA-M9 medium was 6–9 fold less toxic than in Leu-saline (Table 2). The reason for the latter effect has to be further studied.
It is well documented but not so often taken into account in planning of the experiments and interpretation of the results that test medium composition has a strong impact on test results. Traditional Microtox™ test is conducted in 2% NaCl that is a good choice for testing of most of the chemicals. However, as 2% NaCl is not supporting bacterial growth, for chronic toxicity testing it must be supplemented with nutrients. Thus, the choice of the test medium should be made case-by-case taking into account the type of the test and nutritional requirements of test organisms. For example, as seen from our results, even amino-acid supplemented M9 medium could be successfully used in chronic bacterial test for organic compounds.
3.3. Comparative Toxicity of Chemicals to Different Constitutively Luminescent Bacteria
The two bioluminescent E. coli strains chosen for this study, E. coli MC1061(pSLlux) and MC1061(pDNlux) are otherwise identical bacteria but differ by their background bioluminescence, the former being 100-fold more luminescent than the latter. As the bioluminescence of the bacteria is metabolically costly, we assumed that due to the high energetic burden of the bioluminescence, the E. coli MC1061(pSLlux) strain with remarkably higher background luminescence might be more sensitive to the toxicants than E. coli MC1061(pDNlux). To control this hypothesis, toxicity of standard chemicals after 30 min of exposure to both E. coli strains in both, GAA-M9 and Leu-saline were compared (Table 2, Figure 2).
The comparison of the toxicity of the standard chemicals (Table 2) showed that both E. coli strains were of comparable sensitivity in the given test medium. The log-log correlation coefficient of the 30-min EC50 values for these two E. coli strains in GAA-M9 was R2 = 0.97 (p < 0.01) (Figure 2(A)) and in Leu-saline R2 = 0.99 (p < 0.01) (Figure 2(B)). Thus, the 100-fold different background luminescence in these E. coli strains did not have influence on the toxicity test results showing that in the current test conditions the background luminescence was not an “energetic burden” for the cells. Analogously to our toxicity results, it has been previously shown that introducing bioluminescence to a non-luminous Burkholderia sp. did not affect their sensitivity towards Cu and Zn, as evaluated by dehydrogenase activity test [50]. The toxicity data obtained with both luminescent E. coli strains were also compared with V. fischeri (a Microtox™ test strain) (Table 2, Figure 3).
Although chemical-wise the EC50 values for most of the chemicals for E. coli and V. fischeri were statistically different (p < 0.05) except that for 3,5-dichloroaniline (Table 2), the log-log correlation coefficient of the 30-min EC50 values for E. coli (pSLlux) and V. fischeri was R2 = 0.70 (p < 0.05) (Figure 3(A)) and E. coli (pDNlux) and V. fischeri R2 = 0.75 (p < 0.01) (Figure 3(B)). Thus, despite of different test temperatures and slightly different composition of the test environment (2% NaCl vs. 0.005% Leu-supplemented 0.45% NaCl) the 30-min EC50 of E. coli and V. fischeri were in reasonable correlation. Indeed, Deryabin and Aleshina [35] studying the effect of content and nature of different physiological cations and anions on bioluminescence of marine bacterium P. phosphoreum (Microbiosensor B-17 677f) and the recombinant E. coli strain containing lux operon from P. leiognathi showed that factors leading to the formation of luminescent response of these two bacterial strains are universal. Therefore, given that the mechanisms of action of the test chemicals to E. coli and V. fischeri are similar, their bioluminescent response should be comparable.
4. Conclusions
We have shown that Photorhabdus luminescence luxCDABE-transformed constitutively bioluminescent bacteria are promising tools for in vitro toxicology in two aspects: they may replace V. fischeri in tests where the thermostability of the luciferase >30 °C is crucial and they can be powerful screening tools for new antimicrobials.
The kinetic Flash-Assay designed initially for toxicity analysis with naturally luminous V. fischeri proved suitable for artificially luminescent E. coli. In addition, the Flash-Assay (but not the Microtox™ test format) is applicable also for microplate luminometers and high throughput analysis. Moreover, kinetic analysis of the toxic effect of chemicals on bacterial cells may shed light on their mechanisms of toxicity and thus, facilitate the discovery of new disinfectants for clinically and hygienically important environments/surfaces, e.g., potentially contaminated with coliforms.
Last but not least, our study once more confirms the importance of addressing the composition of the test medium, which should be carefully chosen case-by-case depending on the type of the assay and nature of chemicals analyzed.
Acknowledgments
This work was financially supported by the Estonian Ministry of Science and Education (targeted funding project SF0690063s08), Estonian Science Foundation (grants No. 6974, 6956 and 8561), by European Social Fund and by the EU 6th Framework Integrated Project OSIRIS (Contract No. GOCE-CT-2007-037017).
References
- Carere, A; Stammati, A; Zucco, F. In vitro toxicology methods: Impact on regulation from technical and scientific advancements. Toxicol. Lett 2002, 127, 153–160. [Google Scholar]
- Russel, WMS; Burch, RL. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Claxton, LD; Umbuzeiro, GD; Demarini, DM. The Salmonella mutagenicity assay: The stethoscope of genetic toxicology for the 21st century. Environ. Health Perspect 2010, 118, 1515–1522. [Google Scholar]
- Bulich, AA; Isenberg, DL. Use of the luminescent bacterial system for the rapid assessment of aquatic toxicity. ISA Trans 1981, 20, 29–33. [Google Scholar]
- Kaiser, KLE; Devillers, J. Ecotoxicity of Chemicals to Photobacterium Phosphoreum; Handbooks of ecotoxicological data; Gordon and Breach Science Publishers S.A: Amsterdam, The Netherlands, 1994; Volume 2. [Google Scholar]
- Urbanczyk, H; Ast, JC; Higgins, MJ; Carson, J; Dunlap, PV. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol 2007, 57, 2823–2829. [Google Scholar]
- Hastings, JW. Bioluminescence. In Cell Physiology Source Book; Sperelakis, N, Ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Meighen, EA. Molecular biology of bacterial bioluminescence. Microbiol. Rev 1991, 55, 123–142. [Google Scholar]
- Hastings, JW. Bacterial bioluminescence: An overview. In Methods in Enzymology; DeLuca, MA, Ed.; Academic Press: New York, NY, USA, 1978; pp. 125–135. [Google Scholar]
- Kahru, A. In vitro toxicity testing using marine luminescent bacteria Photobacterium phosphoreum: The Biotox™ test. ATLA-Altern. Lab. Anim 1993, 21, 210–215. [Google Scholar]
- Kahru, A; Borchardt, B. Toxicity of 39 MEIC chemicals to bioluminescent photobacteria (the Biotox™ test): Correlation with other test systems. ATLA-Altern. Lab. Anim 1994, 22, 147–160. [Google Scholar]
- Loibner, AP; Szolar, OHJ; Braun, R; Hirmann, D. Toxicity testing of 16 priority polycyclic aromatic hydrocarbons using Lumistox. Environ. Toxicol. Chem 2004, 23, 557–564. [Google Scholar]
- Mortimer, M; Kasemets, K; Kurvet, I; Heinlaan, M; Kahru, A. Kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles and colored/turbid samples. Toxicol. In Vitro 2008, 22, 1412–1417. [Google Scholar]
- Põllumaa, L; Maloveryan, A; Trapido, M; Sillak, H; Kahru, A. Study of the environmental hazard caused by the oil-shale industry solid waste. ATLA-Altern. Lab. Anim 2001, 29, 259–267. [Google Scholar]
- Lapa, N; Barbosa, R; Morais, J; Mendes, B; Méhu, J; Santos Oliveira, JF. Ecotoxicological assessment of leachates from MSWI bottom ashes. Waste Manag 2002, 22, 583–593. [Google Scholar]
- Wang, C; Yediler, A; Lienert, D; Wang, Z; Kettrup, A. Toxicity evaluation of reactive dyestuff, auxiliaries and selected effluents in textile finishing industry to luminescent bacteria Vibrio fisheri. Chemosphere 2002, 46, 339–344. [Google Scholar]
- Manusadzianas, L; Balkelyte, L; Sadauskas, K; Blinova, I; Põllumaa, L; Kahru, A. Ecotoxicological study of Lithuanian and Estonian wastewaters: Selection of the biotests and correspondence between toxicity and chemical-based indices. Aquat. Toxicol 2003, 63, 27–41. [Google Scholar]
- Kaiser, KL. Correlations of Vibrio fischeri bacteria test data with bioassay data for other organisms. Environ. Health Perspect 1998, 106, 583–591. [Google Scholar]
- Kahru, A. Ecotoxicological tests in non-ecotoxicological research: Contribution to the three Rs. Use of luminescent photobacteria for evaluating the toxicity of 47 MEIC reference chemicals. ALTEX 2006, 23, 302–308. [Google Scholar]
- ISO. Water quality—Kinetic Determination of the Inhibitory Effects of Sediment, Other Solids and Coloured Samples on the Light Emission of Vibrio fischeri (Kinetic Luminescent Bacteria Test)— ISO 21338:2010; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Lappalainen, J; Juvonen, R; Nurmi, J; Karp, M. Automated color correction method for Vibrio fischeri toxicity test. Comparison of standard and kinetic assays. Chemosphere 2001, 45, 635–641. [Google Scholar]
- Close, DM; Patterson, SS; Ripp, S; Baek, SJ; Sanseverino, J; Sayler, GS. Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line. PLoS One 2010, 27, e12441. [Google Scholar]
- Westerlund-Karlsson, A; Saviranta, P; Karp, M. Generation of thermostable monomeric luciferases from Photorhabdus luminescens. Biochem. Biophys. Res. Commun 2002, 296, 1072–1076. [Google Scholar]
- Leedjärv, A; Ivask, A; Virta, M; Kahru, A. Analysis of bioavailable phenols from natural samples by recombinant luminescent bacterial sensors. Chemosphere 2006, 64, 1910–1919. [Google Scholar]
- Ivask, A; Rõlova, T; Kahru, A. A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol 2009, 8, 9–41. [Google Scholar]
- Colepicolo, P; Cho, KW; Poinar, GO; Hastings, JW. Growth and luminescence of the bacterium Xenorhabdus luminescens from a human wound. Appl. Environ. Microbiol 1989, 55, 2601–2606. [Google Scholar]
- Francis, KP; Joh, D; Bellinger-Kawahara, C; Hawkinson, MJ; Purchio, TF; Contag, PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun 2000, 68, 3594–3600. [Google Scholar]
- Andreu, N; Zelmer, A; Fletcher, T; Elkington, PT; Ward, TH; Ripoll, J; Parish, T; Bancroft, GJ; Schaible, U; Robertson, BD; et al. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS One 2010, 24, e10777. [Google Scholar]
- Hilpert, K; Hancock, RE. Use of luminescent bacteria for rapid screening and characterization of short cationic antimicrobial peptides synthesized on cellulose using peptide array technology. Nat. Protoc 2007, 2, 1652–1660. [Google Scholar]
- Bondarenko, O; Rahman, PK; Rahman, TJ; Kahru, A; Ivask, A. Effects of rhamnolipids from Pseudomonas aeruginosa DS10–129 on luminescent bacteria: Toxicity and modulation of cadmium bioavailability. Microb. Ecol 2010, 59, 588–600. [Google Scholar]
- Lee, J; Murphy, CL; Faini, GJ; Baucom, TL. Bacterial bioluminescence and its application to analytical procedures. In Liquid Scintillation Counting: Recent Developments; Stanley, PE, Scoggins, BA, Eds.; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Sambrook, J; Fritsch, EF; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Casadaban, MJ; Cohen, SN. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol 1980, 138, 179–207. [Google Scholar]
- Leedjärv, A; Ivask, A; Virta, M; Kahru, A. Analysis of bioavailable phenols from natural samples by recombinant luminescent bacterial sensors. Chemosphere 2006, 64, 1910–1919. [Google Scholar]
- Deryabin, DG; Aleshina, ES. Effect of salts on luminescence of natural and recombinant luminescent bacterial biosensors. Appl. Biochem. Microbiol 2008, 44, 292–296. [Google Scholar]
- Hassan, SH; Oh, SE. Improved detection of toxic chemicals by Photobacterium phosphoreum using modified Boss medium. J. Photochem. Photobiol. B 2010, 101, 16–21. [Google Scholar]
- Zhao, YH; Cronin, MTD; Dearden, JC. Quantitative structure-activity relationships of chemicals acting by non-polar narcosis—theoretical considerations. Quant. Struct.-Act. Relat 1998, 17, 131–138. [Google Scholar]
- Vindimian, E. MSExcel macro REGTOX EV7.0.5.xls. 2005, Available online: http://eric.vindimian.9online.fr/ (accessed on 5 July 2011).
- Nies, DH. Microbial heavy metal resistance. Molecular biology and utilization for biotechnology process. Appl. Microbiol. Biotechnol 1999, 51, 730–750. [Google Scholar]
- Verhaar, HJM; van Leeuwen, CJ; Hermens, JLM. Classifying environmental pollutants. 1: Structure-activity relationships for prediction of aquatic toxicity. Chemosphere 1992, 25, 471–491. [Google Scholar]
- Elnabarawy, MT; Robideau, RR; Beach, SA. Comparison of three rapid toxicity test procedures: Microtox, polytox, and activated sludge respiration inhibition. Toxic. Assess 1988, 3, 361–370. [Google Scholar]
- Dom, N; Knapen, D; Benoot, D; Nobels, I; Blust, R. Aquatic multi-species acute toxicity of (chlorinated) anilines: Experimental versus predicted data. Chemosphere 2010, 81, 177–186. [Google Scholar]
- Ribo, JM; Yang, JE; Huang, PM. Luminescent bacteria toxicity assay in the study of mercury speciation. Hydrobiologia 1989, 188/189, 155–162. [Google Scholar]
- Utgikar, VP; Chaudhary, N; Koeniger, A; Tabak, HH; Haines, JR; Govind, R. Toxicity of metals and metal mixtures: Analysis of concentration and time dependence for zinc and copper. Water Res 2004, 38, 3651–3658. [Google Scholar]
- Villaescusa, I; Pilar, M; Hosta, C; Martinez, M; Murat, JC. Toxicity of cadmium species on luminescent bacteria. Fresenius’ J. Anal. Chem 1996, 354, 566–570. [Google Scholar]
- Denich, TJ; Beaudette, LA; Lee, H; Trevors, JT. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar]
- Zhang, YM; Rock, CO. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol 2008, 6, 222–233. [Google Scholar]
- Gellert, G. Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol. Environ. Saf 2000, 45, 87–91. [Google Scholar]
- Riether, KB; Dollard, MA; Billard, P. Assessment of heavy metal bioavailability using Escherichia coli zntAp::lux and copAp::lux-based biosensors. Appl. Microbiol. Biotechnol 2001, 57, 712–716. [Google Scholar]
- Chinalia, FA; Paton, GI; Killham, KS. Physiological and toxicological characterization of an engineered whole-cell biosensor. Bioresour. Technol 2008, 99, 714–721. [Google Scholar]



| E. coli strain | Description | Antibiotic added | References |
|---|---|---|---|
| MC1061 (pDNlux) | (araD139 Δ(ara, leu)7697 Δ lacX74 galU galK hsdR2 strA mcrA mcrB1)a luxCDABE genes from Photorhabdus luminescens in a medium-copy plasmid pDNlux b | Tetracycline (10 mg/L) (Boehringer-Mannheim GmbH, Mannheim, Germany) | [25] |
| MC1061 (pSLlux) | Same as above but luxCDABE genes in a high-copy plasmid pSLlux c | Ampicillin (100 mg/L) (Serva, Feinbiochemika, Heidelberg/New York) | [25] |
adefective for the synthesis of leucine [33];bTcr, luxCDABE in pDN18N constructed in [34] were electroporated into E. coli MC1061 to obtain constitutively luminescent strain E. coli MC1061(pDNlux);cApr, luxCDABE in pSL1190 constructed in [34] were electroporated into E. coli MC1061 to obtain constitutively luminescent strains E. coli MC1061(pSLlux).
| Chemical | E. coli MC1061(pDNlux) | E. coli MC1061(pSLlux) | V. fischeri | ||
|---|---|---|---|---|---|
| Test medium: | GAA-M9 a | Leu-saline b | GAA-M9 a | Leu-saline b | 2% saline c |
| Test temperature: | 30 °C | 30 °C | 30 °C | 30 °C | 20 °C |
| Zn2+ (ZnSO4·7H2O) | 1,089 ± 147 | 0.60 ± 0.083 d | 1,009 ± 114 | 0.69 ± 0.46 d | 9.27 ± 0.38 e |
| Cd2+ (CdCl2·H2O) | 345 ± 1.47 | 1.94 ± 1.01 d | 1,085 ± 441 | 3.49 ± 1.51 d | 31 ± 18.2 e |
| Hg2+ (HgCl2) | 0.39 ± 0.09 | 0.04 ± 0.002 d | 1.9 ± 0.44 | 0.049 ± 0.02 d | 0.23 ± 0.13 e |
| Cu2+ (CuSO4) | 83.5 ± 10.9 | 1.22 ± 0.94 d | 131 ± 20.1 | 3.26 ± 0.88 d | 0.30 ± 0.15 e |
| 3,5-dichlorophenol (3,5-DCP) | 14.8 ± 4.04 | 2.29 ± 0.55 d | 26.9 ± 5.12 | 3.0 ± 0.28 d | 4.38 ± 0.98 e |
| Aniline | 1,362 ± 43.5 | 1,251 ± 39.9 | 2,136 ± 64.1 | 1,683.5 ± 388 | 552.4 ± 151.3 e |
| 3,5-dichloroaniline (3,5-DCA) | 47.6 ± 7.34 | 49.8 ± 5.87 | 56.7 ± 8.16 | 51.14 ± 7.2 | 47.59 ± 12.8 |
a50% M9 medium supplemented with 0.05% glucose and 0.25% cas-aminocids;b0.45% NaCl supplemented with 0.001% leucine;c2% NaCl;dstatistically different (p < 0.05) from the EC50 value in GAA-M9 for the same chemical and the same bacterial strain;estatistically different (p < 0.05) from the EC50 value of the E. coli in Leu-saline for the same chemical.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurvet, I.; Ivask, A.; Bondarenko, O.; Sihtmäe, M.; Kahru, A. LuxCDABE—Transformed Constitutively Bioluminescent Escherichia coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio fischeri. Sensors 2011, 11, 7865-7878. https://doi.org/10.3390/s110807865
Kurvet I, Ivask A, Bondarenko O, Sihtmäe M, Kahru A. LuxCDABE—Transformed Constitutively Bioluminescent Escherichia coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio fischeri. Sensors. 2011; 11(8):7865-7878. https://doi.org/10.3390/s110807865
Chicago/Turabian StyleKurvet, Imbi, Angela Ivask, Olesja Bondarenko, Mariliis Sihtmäe, and Anne Kahru. 2011. "LuxCDABE—Transformed Constitutively Bioluminescent Escherichia coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio fischeri" Sensors 11, no. 8: 7865-7878. https://doi.org/10.3390/s110807865