A Fast Response Highly Selective Probe for the Detection of Glutathione in Human Blood Plasma
Abstract
: A fluorescent probe for glutathione (GSH) detection was developed. Our study indicates a possible mechanism which couples a conjugate addition and micelle-catalyzed large membered ring formation/elimination sequence. This method enables excellent selectivity towards GSH over other biological thiols such as cysteine (Cys) and homocysteine (Hcy). The proposed method is precise with a relative standard deviation (R.S.D) lower than 6% (n = 3) and has been successfully applied to determine GSH in human plasma with recoveries between 99.2% and 102.3%.1. Introduction
Glutathione (GSH) serves as an antioxidant and an important indicator of cellular oxidative stress [1]. Aberrant levels of GSH have been associated with a number of diseases, including cancer, AIDS, Alzheimer's and cardiovascular disease [2]. Fluorescent and colorimetric probes for the detection of thiols have been widely reported [3]. However, indicators that are selective for GSH and not generally selective for sulfhydril-containing compounds are relatively rare. Although several recent papers claim GSH selectivity, the indicators display significant responses to Cys and other related nucleophilic thiols [4–7]. A promising GSH-selective probe for selective intracellular imaging applications has been developed by Shao et al. [8]. In addition, innovative nanoparticle or polymeric indicators for GSH also exhibit high selectivity, but to date they have not been successfully used in biological media, as they either are based on toxic CdSe or require the handling of highly toxic mercury salts to function [9,10].
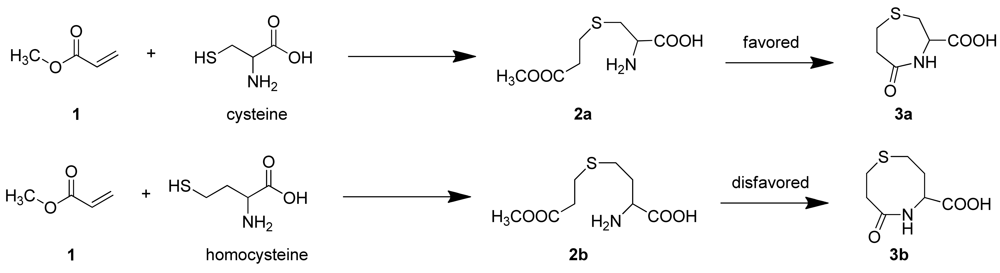
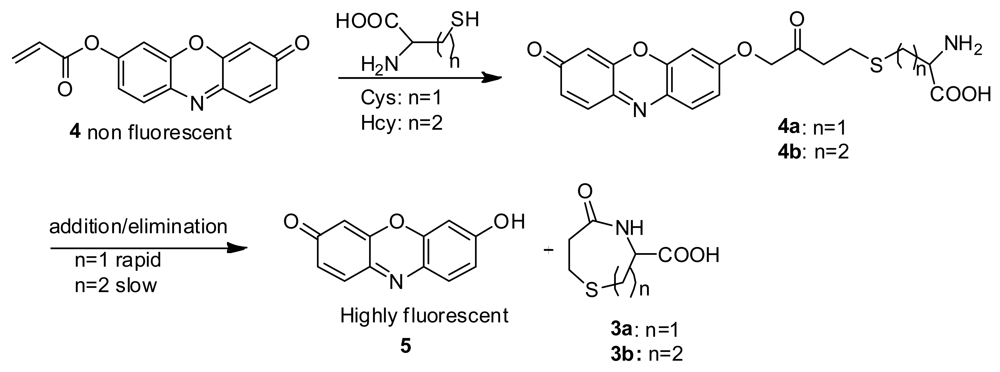
It has been reported that Cys reacts with acrylates to generate thioethers that undergo an intramolecular cyclization reaction to yield, for example, 3-carboxy-5-oxoperhydro-1,4-thiazepine (3a) [11]. In the case of Hcy, 2b should be easily obtained [12], however, the intramolecular cyclization reaction to form a eight-membered ring (3b) is kinetically disfavored, compared with the formation of seven membered ring (3a) which would result from Cys (Scheme 1) [13–15].
Recently, a new design for a fluorescent probe capable of distinguishing Cys and Hcy was developed in our lab. A (hydroxymethoxyphenyl)benzothiazole (HMBT)-based probe functioned based on a combined photo-induced electron transfer (PET) and excited-state intramolecular proton transfer (ESIPT) mechanism [16]. More recently, we developed a seminaphthofluorescein (SNF)-based probe for the long wavelength, highly selective detection of Cys. It couples a conjugate addition/cyclization mechanism to a xanthene dye spirolactone-opening reaction [17].
However, to achieve the highly selective detection of GSH over Cys and Hcy, one would need to overcome the formidable challenge of favoring the formation of a larger (12-membered ring in the case of GSH) over a 7- and 8-membered ring (as in 3a and 3b). Interestingly, it had been reported earlier that cetyltrimethylammonium bromide (CTAB) micelles can catalyze the intramolecular ring closure of larger rings, reversing the expected kinetics based on ring size [18]. It is also known that GSH binds the surface of cationic CTAB micelle [19]. We reasoned that a relatively smaller and more planar dye as compared to the HMBT and SNF probes used previously may interact more with the micelle and thus promote highly selective detection of GSH [20]. We thus herein report the successful use of a resorufin-based probe for the highly selective detection of GSH in CTAB medium.
2. Experimental Section
2.1. Materials and Instruments
All chemicals were purchased from Sigma-Aldrich or Acros and used without further purification.1H-NMR and 13C-NMR spectra were recorded on a Bruker AMX-400 NMR spectrometer, using TMS as an internal standard. ESI-HRMS (high resolution mass spectrometry) spectra were obtained on a Thermo Electron LTQ Orbitrap hybrid mass spectrometer. UV-visible spectra were collected on a Cary 50 UV-Vis spectrophotometer; Fluorescence spectra were collected on a Cary Eclipse (Varian, Inc.) fluorescence spectrophotometer with slit widths set at 5 nm for both excitation and emission, respectively. The high voltage of the fluorescence spectrophotometer was set at 500 V for the resorufin-based probe 4. The pH measurements were carried out with an Orion 410A pH meter.
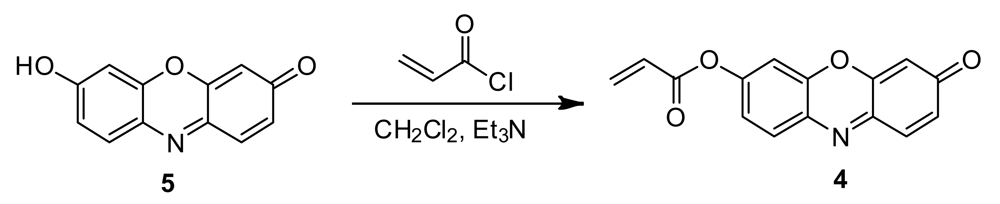
2.2. Synthesis of Probe 4
Probe 4 was synthesized in a one-step reaction of resorufin with acryloyl chloride (Scheme 2). To a solution of resorufin (220 mg) and Et3N (1.5 equiv) in 15 mL of anhydrous CH2Cl2, acryloyl chloride (2.0 equiv in 5 mL of CH2Cl2) is added dropwise at 0 °C. After stirring at this temperature for 60 min, the resulting mixture is allowed to cool to room temperature and stirred overnight. The mixture is diluted with CH2Cl2 (20 mL), washed with H2O (10 mL × 3) and dried over anhydrous Na2SO4. The solvent is removed by evaporation to afford an orange solid (172 mg, 70% yield). 1H NMR (CDCl3, 400 MHz), δ (ppm): 7.92 (d, 1H, J = 8.7 Hz), 7.59 (d, 1H, J = 9.8 Hz), 7.49 (s, 1H), 7.33 (dd, 1H, J1 = 2.4 Hz, J2 = 2.5 Hz), 6.83 (dd, 1H, J1 = 2.0 Hz, J2 = 2.0 Hz), 6.63 (d, 1H, J = 17.2 Hz), 6.49 (m, 1H), 6.24 (s, 1H), 6.22 (d, 1H, J = 1.0 Hz). 13C NMR (CDCl3, 100 MHz), δ 186.48, 163.84, 153.24, 148.87, 147.78, 143.55, 134.85, 134.26, 134.15, 131.22, 131.16, 127.18, 118.82, 109.94, 105.86. ESI-MS m/z = 268.0833 [M+H]+, calc. 268.0810 for C15H10NO4.
3. Results and Discussion
3.1. Intrinsic Cysteine Selectivity in the Absence of Surfactant
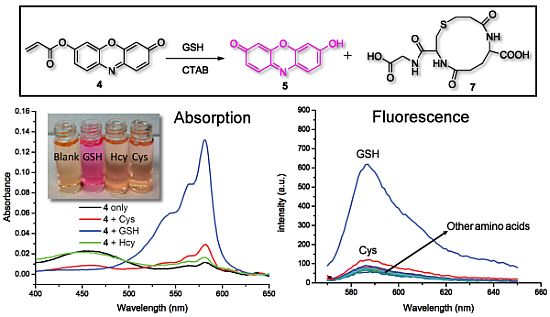
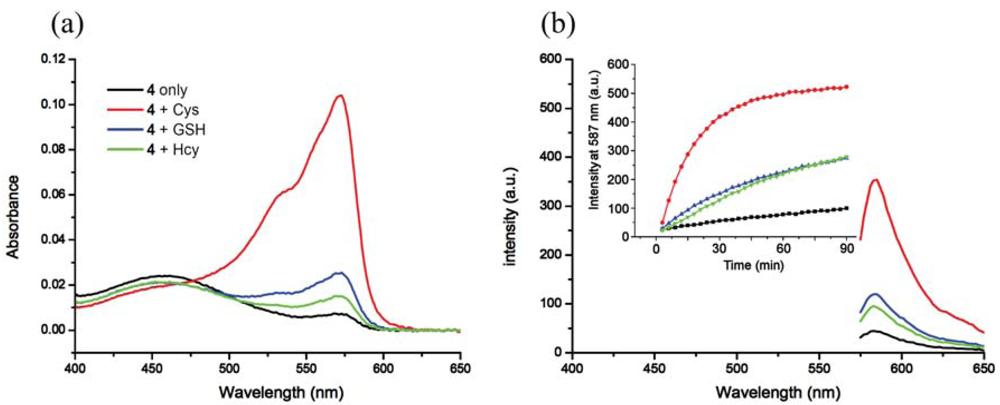
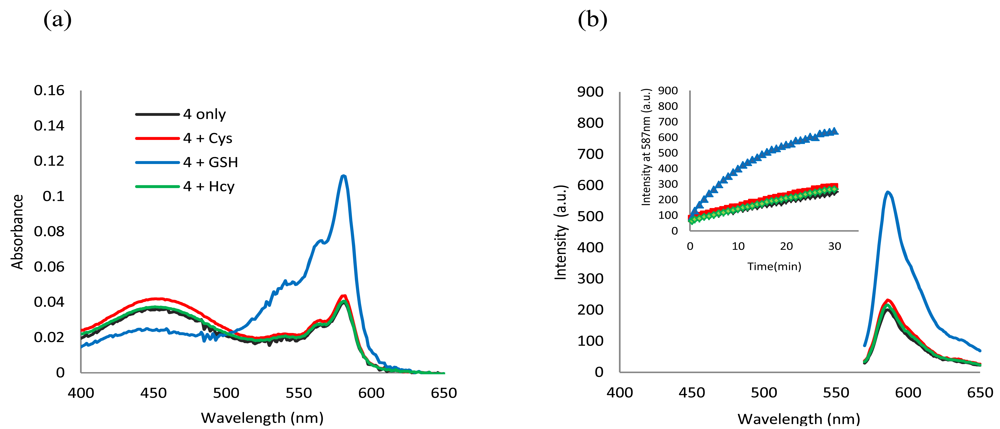
In buffer without a surfactant, as expected, 4 shows excellent selectivity towards Cys due to the reported kinetically favored 7-membered ring formation. Upon mixing Cys with 4, the conjugate addition product 4a is formed, which undergoes a rapid cyclization reaction to produce 3a while releasing the free resorufin (Scheme 3). A significant color change and fluorescence enhancement can be observed in response to Cys (Figure 1). In the case of Hcy, because it has an additional methylene group in its side chain, a kinetically less favored 8-membered ring would form. As for GSH, 1,4-addition of thiols to the α,β-unsaturated carbonyl moieties of 4 can occur readily; however, the ensuing intramolecular cyclization similar to that of Cys cannot proceed without the presence of surfactant. No significant color formation or fluorescence response is seen in the case of Hcy or GSH under these conditions.
The time course of the fluorescence assay is shown in Figure 1(b). It can be seen that the fluorescence upon reaction with Cys increases with time and reaches a plateau after about 90 min, whereas for Hcy and GSH the reactions are significantly slower. The sensitivity and linearity of the response of 4 towards Cys was also investigated. Linear response with submicromolar sensitivity was observed (See Figure S5 in the Electronic Supplementary Information). The reaction products between 4 and thiols were further studied using HRMS (See Figures S6 and S7 in the Electronic Supplementary Information).
3.2. Surfactant Mediated Modification of the Intrinsic Selectivity
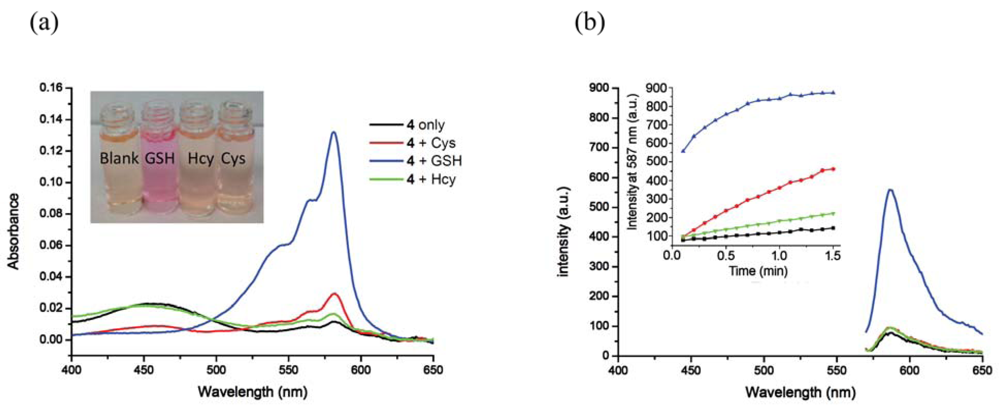
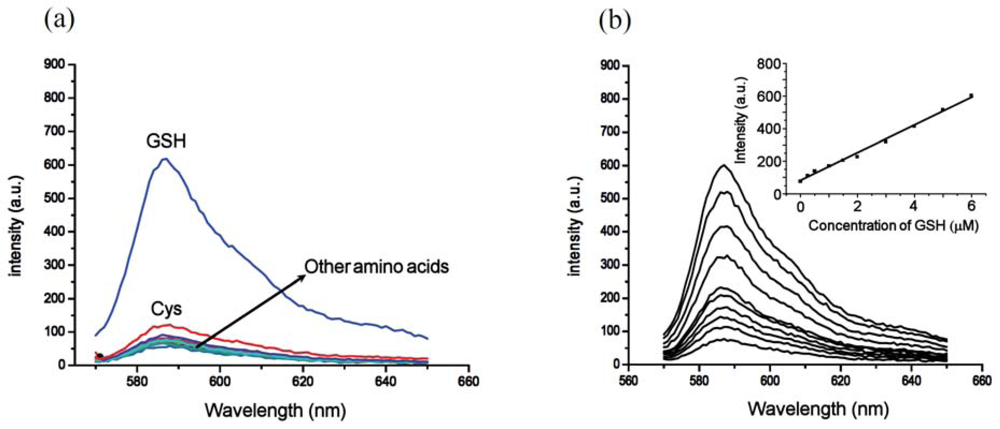
Inclusion of surfactants (see Figure 2) dramatically alters the intrinsic selectivity of 4. For example, solutions of GSH, Cys and Hcy (2 equiv) with the resorufin-acrylate probe 4 in pH 7.4 phosphate buffer generate the characteristic strong pink color immediately upon addition of CTAB only in the presence of GSH (Figure 3(a)). Other amino acids, such as Hcy and Cys, did not exhibit significant changes under the same conditions. This interesting feature indicates that 4-CTAB system can serve as a selective visual inspection dosimeter for GSH. Corresponding fluorescence increases were also observed (Figure 3(b)). Fluorescence upon reaction with GSH reaches a plateau in less than 2 min.
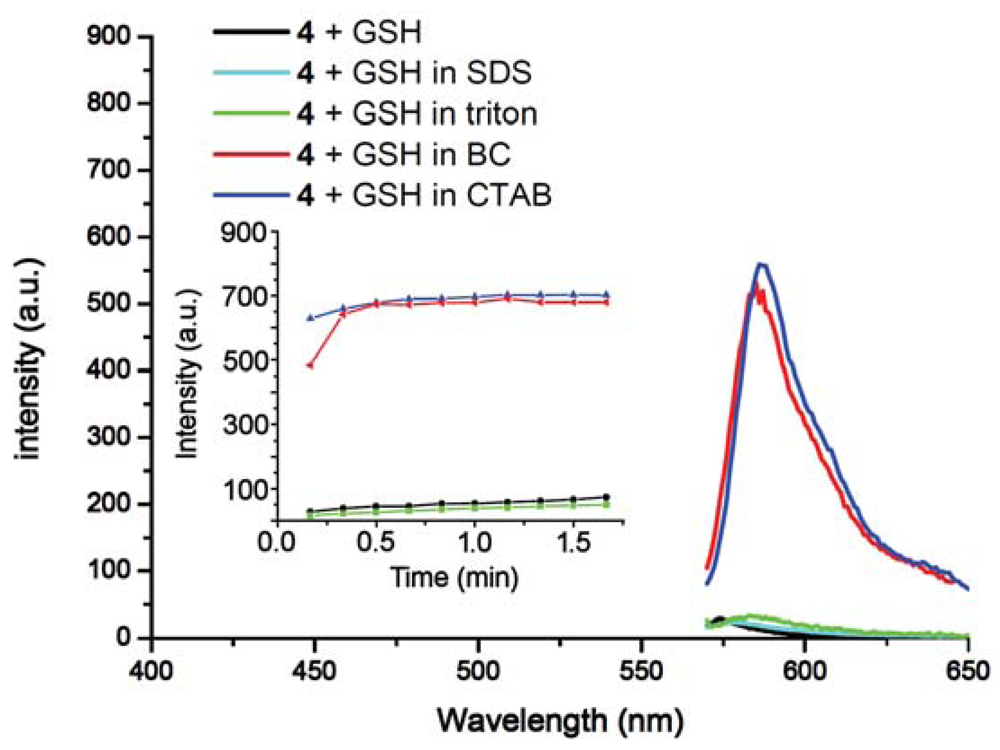
To study the effects of different surfactants, SDS, BC and Triton X-100 were evaluated. It was found that 4 serves as an outstanding indicator for GSH only in the presence of cationic surfactants such as BC and CTAB (Figure 4). Negatively charged SDS suppressed the response of 4 towards all three thiols; while the non-ionic surfactant Triton X-100 enhanced the selectivity towards Cys resulting in zero response for Hcy or GSH (See Figure S8 in the Electronic Supplementary Information). The result indicates that selectivity of 4 is tunable by simply changing the reaction media rather than the chromogen.
To further evaluate the selectivity of the 4-CTAB system towards GSH, control experiments using a series of other amino acids were performed measuring the fluorescence responses. Other amino acids produced no significant fluorescence enhancement as compared to GSH, further demonstrating the excellent selectivity for GSH (Figure 5(a)). Moreover, significant fluorescence enhancement can still be observed for GSH even in the presence of excess Cys (see Figure S9 in the Electronic Supplementary Information). Additionally, the fluorescence enhancement of the 4-CTAB system displayed submicromolar sensitivity and a linear relationship with the concentration of GSH over a wide range (Figure 5(b)). The system functions well within the range of typical GSH concentrations in human plasma [21].
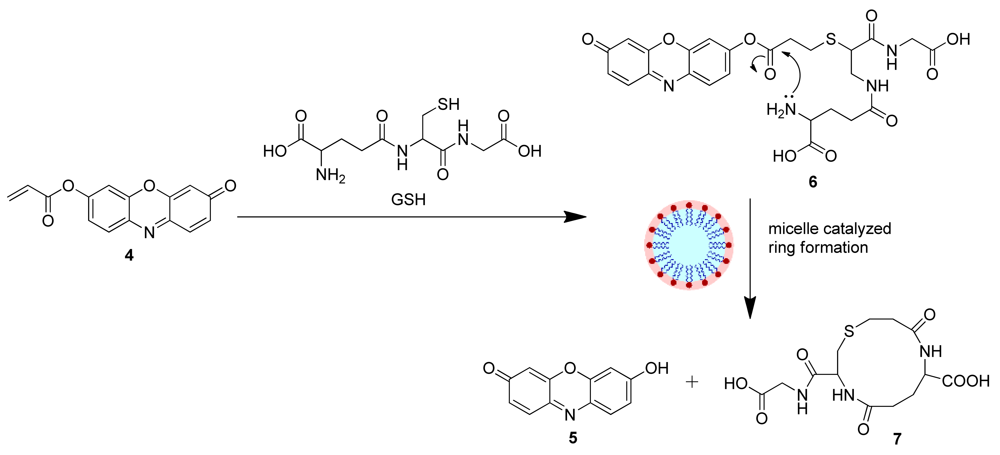
Based on the above results, we propose a plausible mechanism for the GSH selectivity of the 4-CTAB system that involves the conjugate addition of GSH to 4 to generate 6, which in turn undergoes an intramolecular cyclization/elimination reaction sequence catalyzed by the CTAB micelle releasing the free resorufin dye (Scheme 4), resulting in the recovery of the fluorescence. Formation of the 12-membered ring (7) and the free resorufin dye were further investigated via HRMS (See Figures S10, S11 in the Electronic Supplementary Information).
3.3. Determination of GSH in Human Plasma Using the 4-CTAB System
While the 4-CTAB-GSH signal is high at pH 7.4 (see above), it is known that acrylate esters are susceptible to hydrolysis at high pH and that GSH is also relatively unstable under the same conditions (half-life of 9 hours at pH 7.4 compared to 16 hours at pH 6.5) [22]. In this regard, spectral behavior of the 4-CTAB system was investigated over a wide range of pH values (pH 5.5 to pH 8) and its response to the various thiols was studied at lower pH values. As expected, the acrylate-based probe 4 showed the greatest background signal at the higher pH values investigated. The 4-CTAB system was most stable at lower pH values (See Figure S12 in the Electronic Supplementary Information). The reaction with thiols at pH 6 is slightly less rapid as compared to pH 7.4; however, the selectivity towards GSH was further enhanced through the complete elimination of any residual Cys and Hcy response (Figure 6). Thus pH 6 was chosen for further application studies in plasma.
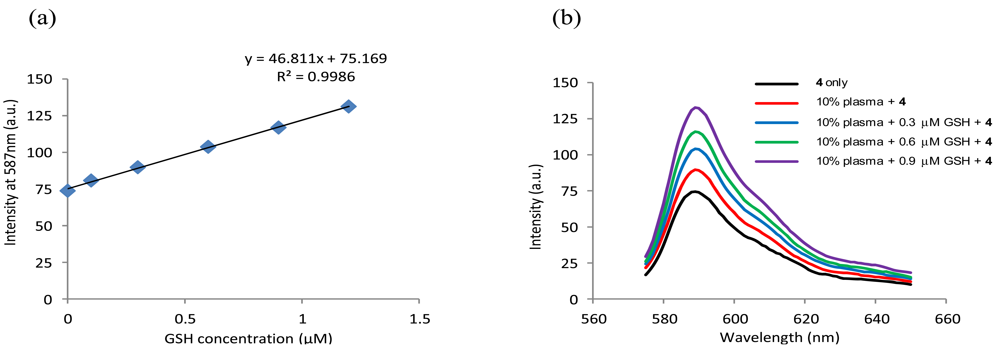
The detection of GSH was performed in 10% deproteinized human plasma. Plasma proteins were precipitated using MeCN (two thirds of the reconstitution volume) and removed by centrifugation at 4,000 rpm for 30 min. The supernatant liquid was diluted [23] and added to a solution of 4 (1 μM) in phosphate buffer (pH 6, 50 mM) in the presence of 2.0 mM CTAB. The fluorescence response of replicate (n = 3) samples and GSH spiked samples were monitored as above and the GSH content in the plasma sample was determined from the regression equation of a standard calibration curve (Figure 7). The GSH content of the plasma sample was determined to be 3.24 ± 0.14 μM, which is well within the reported GSH concentration range for human plasma samples from healthy individuals [21]. Recoveries of the known spiked amounts of GSH were between 99.2% and 102.3% with a generally satisfactory precision (Table 1). These results revealed the potential applicability and reliability of using 4 in quantitative detection of GSH in human plasma. In addition, Cys exhibited significant interference with the GSH determination (See Figure S13 in the Electronic Supplementary Information).
4. Conclusions
Probe 4 can function as a simple new indicator for the highly selective and sensitive detection of GSH. This embodies a sensing mechanism that couples a conjugate addition reaction and a micelle-promoted 12-membered ring formation/elimination sequence. Due to the lack of fluorescence probes for the highly selective detection of GSH over other thiols in blood plasma without the use of biohazardous materials, the discovery of 4 is highly significant. A new class of fluorescent probes for GSH may be developed through this new mechanism. Besides, the method can be tuned to afford Cys selectivity if needed as well.
Supplementary Material
sensors-12-05940-s001.pdfAcknowledgments
This work was supported by the National Institutes of Health (RO1 EB002044).
References
- Miller, E.W.; Bian, S.X.; Chang, C.J. A fluorescent sensor for imaging reversible redox cycles in living cells. J. Am. Chem. Soc. 2007, 129, 3458–3459. [Google Scholar]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar]
- Xu, J.P.; Song, Z.G.; Fang, Y.A.; Mei, J.; Jia, L.; Qin, A.J.; Sun, J.Z.; Ji, J.A.; Tang, B.Z. Label-free fluorescence detection of mercury(II) and glutathione based on Hg(2+)-DNA complexes stimulating aggregation-induced emission of a tetraphenylethene derivative. Analyst 2010, 135, 3002–3007. [Google Scholar]
- Lu, C.; Li, Q.Q.; Chen, S.; Zhao, L.X.; Zheng, Z.X. Gold nanorod-catalyzed luminol chemiluminescence and its selective determination of glutathione in the cell extracts of Saccharomyces cerevisiae. Talanta 2011, 85, 476–481. [Google Scholar]
- Kim, G.J.; Lee, K.; Kwon, H.; Kim, H.J. Ratiometric fluorescence imaging of cellular glutathione. Org. Lett. 2011, 13, 2799–2801. [Google Scholar]
- Ahn, Y.H.; Lee, J.S.; Chang, Y.T. Combinatorial rosamine library and application to in vivo glutathione probe. J. Am. Chem. Soc. 2007, 129, 4510–4511. [Google Scholar]
- Shao, N.; Jin, J.Y.; Wang, H.; Zheng, J.; Yang, R.H.; Chan, W.H.; Abliz, Z. Design of bis-spiropyran ligands as dipolar molecule receptors and application to in vivo glutathione fluorescent probes. J. Am. Chem. Soc. 2010, 132, 725–736. [Google Scholar]
- Li, Y.; Wu, P.; Xu, H.; Zhang, H.; Zhong, X.H. Anti-aggregation of gold nanoparticle-based colorimetric sensor for glutathione with excellent selectivity and sensitivity. Analyst 2011, 136, 196–200. [Google Scholar]
- Tian, D.; Qian, Z.; Xia, Y.; Zhu, C. Gold nanocluster-based fluorescent probes for near-infrared and turning-on sensing of glutathione in living cells. Langmuir 2012, 28, 3945–3951. [Google Scholar]
- Blondeau, P.; Gauthier, R.; Berse, C.; Gravel, D. Synthesis of some stable 7-halo-1,4-thiazepines potential substituted penam precursors. Can. J. Chem. 1971, 49, 3866–3876. [Google Scholar]
- Khatik, G.L.; Kumar, R.; Chakraborti, A.K. Catalyst-free conjugated addition of thiols to alpha,beta-unsaturated carbonyl compounds in water. Org. Lett. 2006, 8, 2432–2436. [Google Scholar]
- Illuminati, G.; Mandolini, L. Ring closure reactions of bifunctional chain molecules. Acc. Chem. Res. 1981, 14, 95–102. [Google Scholar]
- Galli, C.; Mandolini, L. The role of ring strain on the ease of ring closure of bifunctional chain molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125. [Google Scholar]
- Galli, C.; Illuminati, G.; Mandolini, L.; Tamborra, P. Ring closure reactions. 7. Kinetics and activation parameters of lactone formation in range of 3-membered to 23-membered rings. J. Am. Chem. Soc. 1977, 99, 2591–2597. [Google Scholar]
- Yang, X.; Guo, Y.; Strongin, R.M. Conjugate addition/cyclization sequence enables selective and simultaneous fluorescence detection of cysteine and homocysteine. Angew. Chem. Int. Ed. 2011, 50, 10690–10693. [Google Scholar]
- Yang, X.; Guo, Y.; Strongin, R.M. A seminapthofluorescein-based fluorescent chemodosimeter for the highly selective detection of cysteine. Org. Biomol. Chem. 2012, 10, 2739–2741. [Google Scholar]
- Wei, L.; Lucas, A.; Yue, J.; Lennox, R.B. Enhanced cyclization rates of large rings induced by a micellar environment. Langmuir 1991, 7, 1336–1339. [Google Scholar]
- Huang, X.; Dong, Z.Y.; Liu, J.Q.; Mao, S.Z.; Xu, J.Y.; Luo, G.M.; Shen, J.C. Selenium-mediated micellar catalyst: An efficient enzyme model for glutathione peroxidase-like catalysis. Langmuir 2007, 23, 1518–1522. [Google Scholar]
- Bachofer, S.J.; Simonis, U.; Nowicki, T.A. Orientational binding of substituted naphthoate counterions to the tetradecyltrimethylammonium bromide micellar interface. J. Phys. Chem. 1991, 95, 480–488. [Google Scholar]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar]
- Stevens, R.; Stevens, L.; Price, N.C. The stabilities of various thiol compounds used in protein purifications. Biochem. Educ. 1983, 11, 70. [Google Scholar]
- Tang, B.; Liu, F.; Xu, K.; Tong, L. A novel metallobridged bis(β-cyclodextrin)s fluorescent probe for the determination of glutathione. FEBS J. 2008, 275, 1510–1517. [Google Scholar]

| Sample | GSH Spiked (μM) | GSH Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 10% Plasma | 0 | 0.324 | - | 4.42 |
| 10% Plasma + GSH | 0.3 | 0.631 | 102.3 | 4.90 |
| 10% Plasma + GSH | 0.6 | 0.919 | 99.2 | 4.24 |
| 10% Plasma + GSH | 0.9 | 1.224 | 100.0 | 5.88 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, Y.; Yang, X.; Hakuna, L.; Barve, A.; Escobedo, J.O.; Lowry, M.; Strongin, R.M. A Fast Response Highly Selective Probe for the Detection of Glutathione in Human Blood Plasma. Sensors 2012, 12, 5940-5950. https://doi.org/10.3390/s120505940
Guo Y, Yang X, Hakuna L, Barve A, Escobedo JO, Lowry M, Strongin RM. A Fast Response Highly Selective Probe for the Detection of Glutathione in Human Blood Plasma. Sensors. 2012; 12(5):5940-5950. https://doi.org/10.3390/s120505940
Chicago/Turabian StyleGuo, Yixing, Xiaofeng Yang, Lovemore Hakuna, Aabha Barve, Jorge O. Escobedo, Mark Lowry, and Robert M. Strongin. 2012. "A Fast Response Highly Selective Probe for the Detection of Glutathione in Human Blood Plasma" Sensors 12, no. 5: 5940-5950. https://doi.org/10.3390/s120505940