Measurement of Urinary N-Telopeptides and Serum C-Telopeptides from Type I Collagen Using a Lateral Flow-Based Immunoassay
Abstract
:1. Introduction
2. Experimental Section
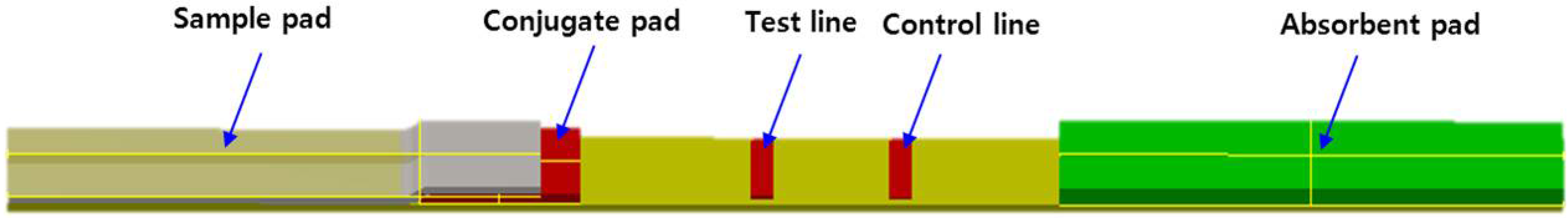
2.1. Development of the Strip for Lateral Flow Based Immunoassay
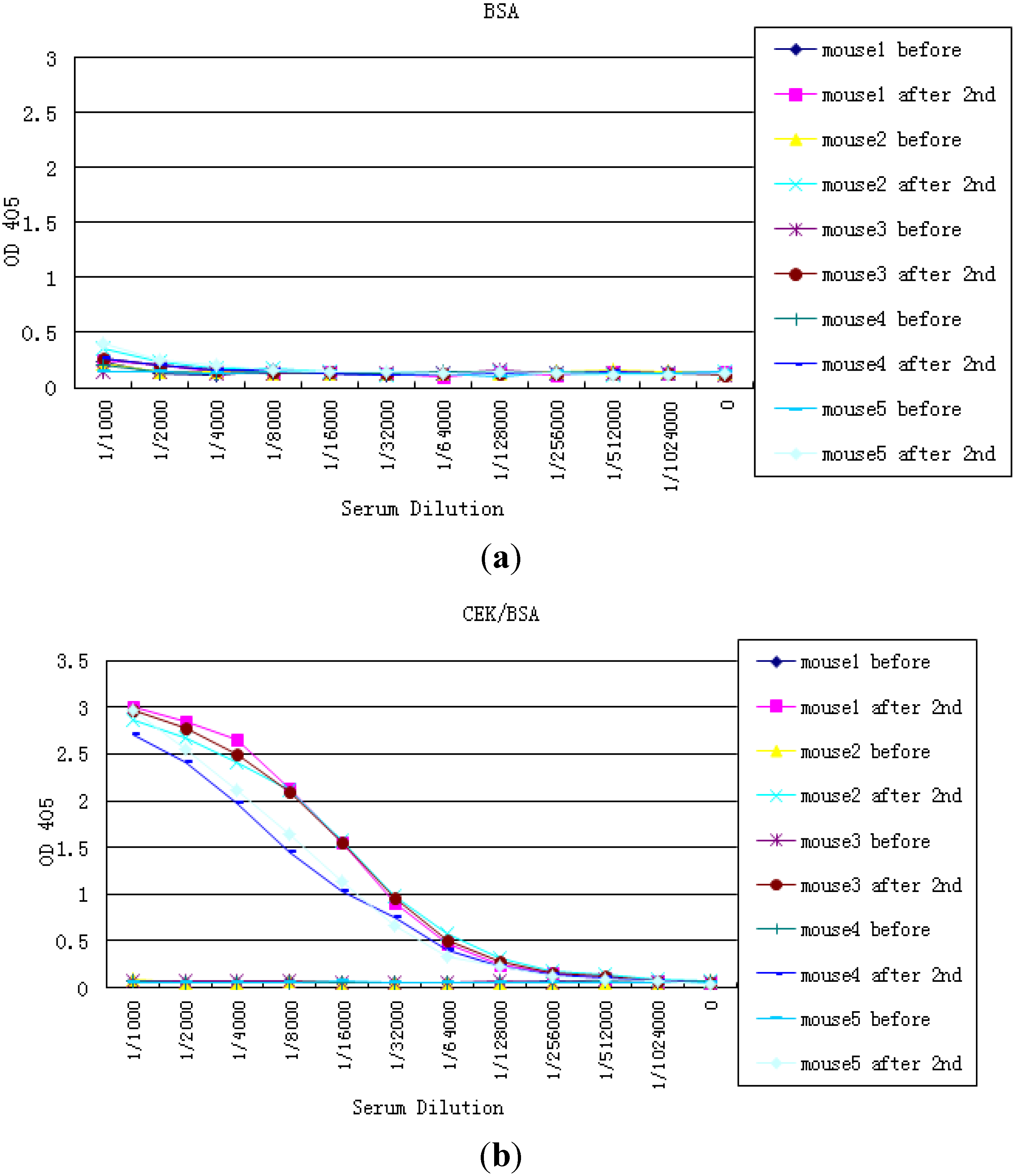
2.2. Preparation NTx and CTx Conjugates
2.3. Preparation of the Strip Assembly
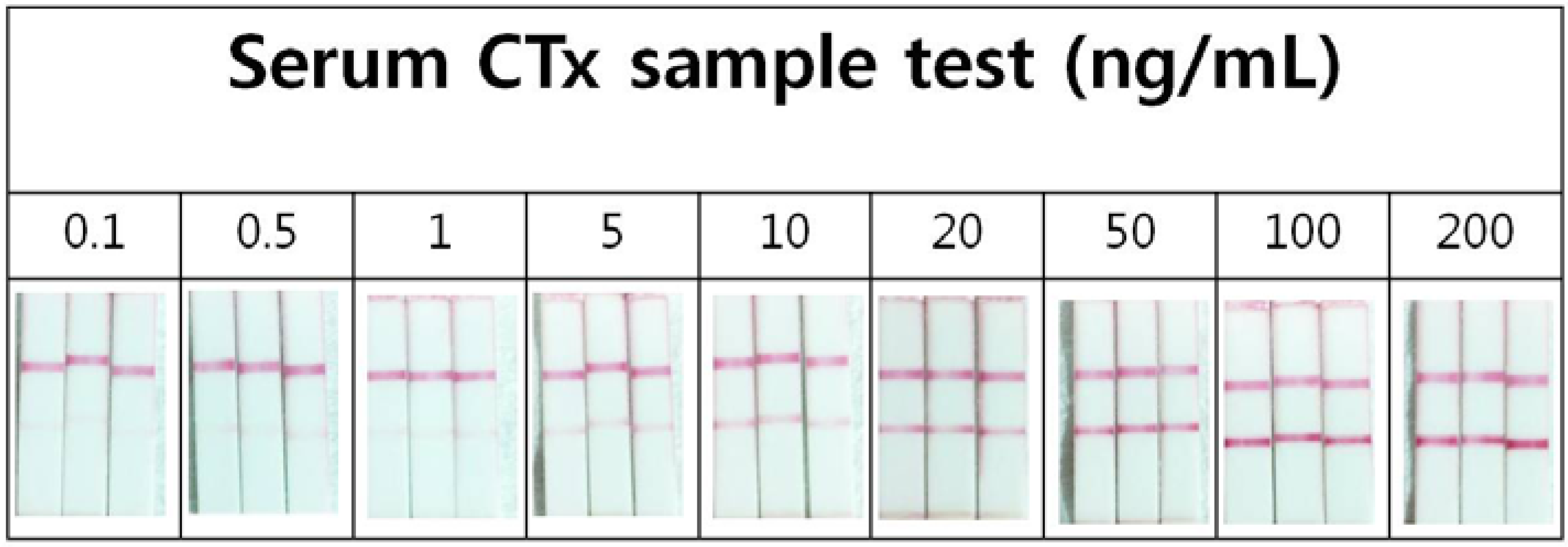
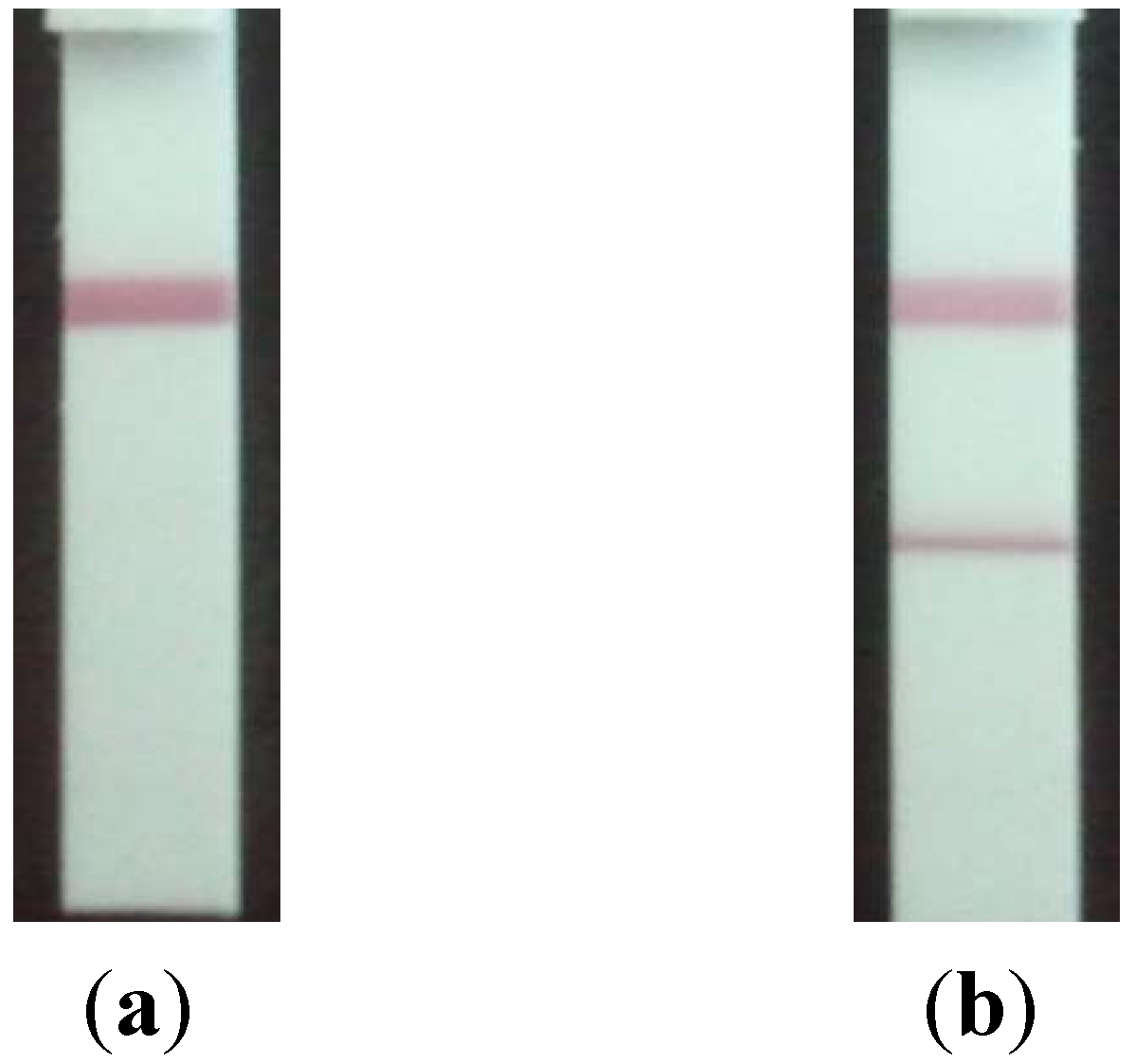
2.4. Precision of the Lateral Flow Based Immunoassay for Repetitively Measuring Urinary NTx and Serum CTx Levels
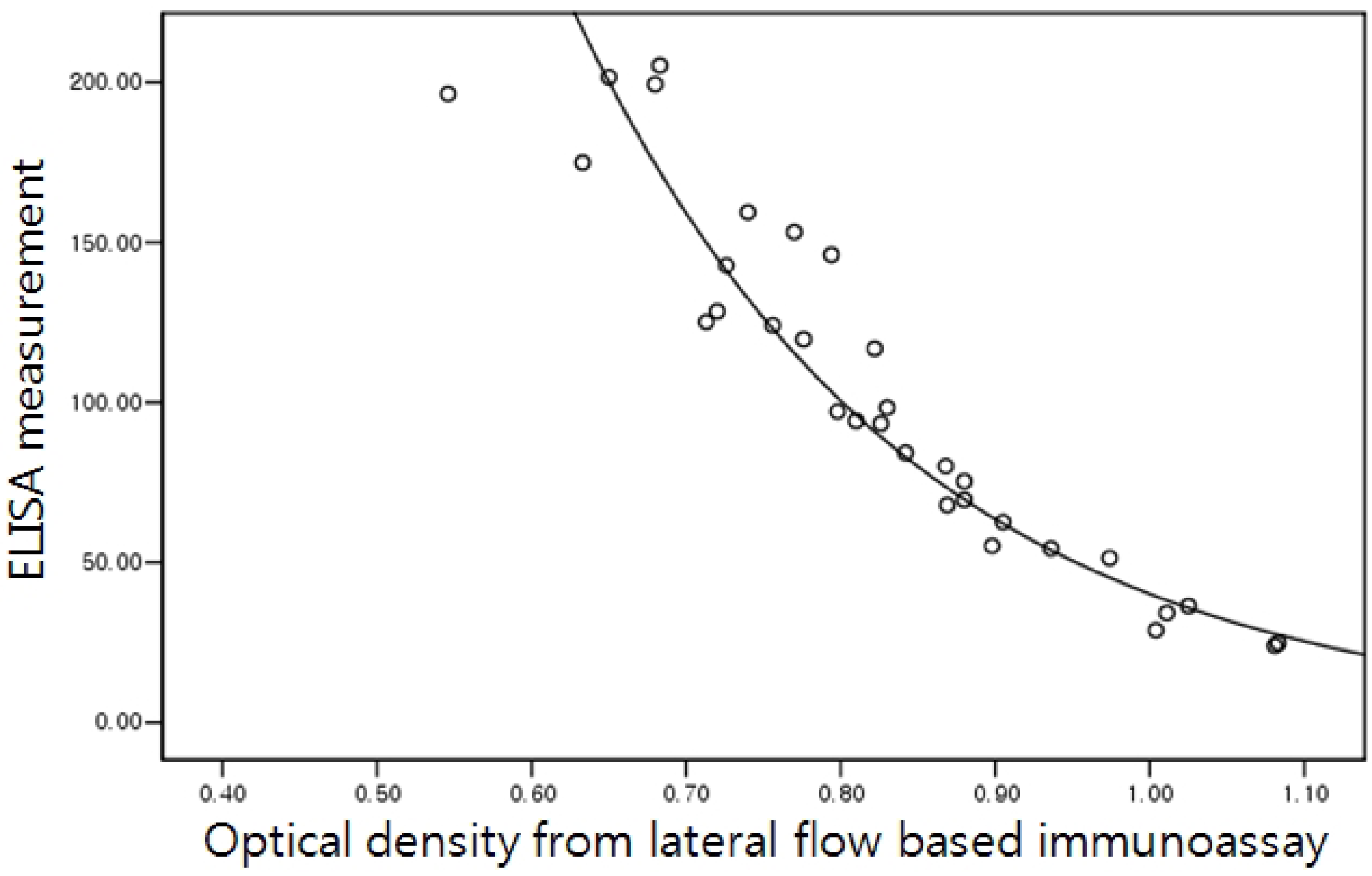
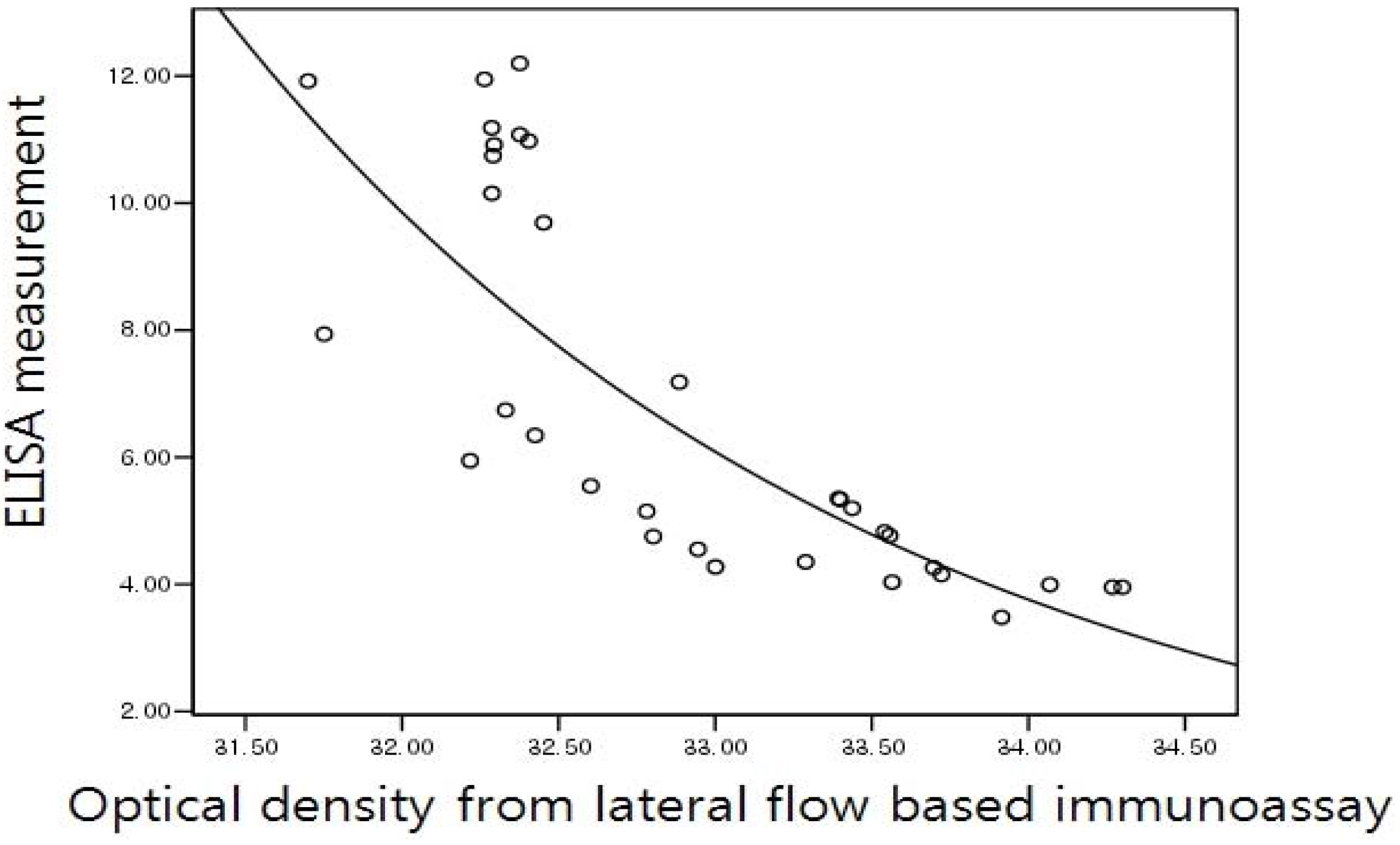
2.5. Correlation of the Lateral Flow-Based Immunoassay for Measuring Urinary NTx and Serum CTx with ELISA Measurements
2.6. Statistical Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Grey, A.; Bolland, M.J. The effect of treatments for osteoporosis on mortality. Osteoporos. Int. 2012, in press. [Google Scholar]
- Cummings, S.R.; Black, D. Bone mass measurements and risk of fracture in Caucasian women: A review of findings from prospective studies. Am. J. Med. 1995, 98, 24S–28S. [Google Scholar]
- Johnell, O.; Gullberg, B.; Kanis, J.A.; Allander, E.; Elffors, L.; Dequeker, J.; Dilsen, G.; Gennari, C.; Lopes, V.A.; Lyritis, G.; et al. Risk factors for hip fracture in European women: The MEDOS Study. Mediterranean Osteoporosis Study. J. Bone. Miner. Res. 1995, 10, 1802–1815. [Google Scholar]
- Cummings, S.R.; Black, D.M.; Nevitt, M.C.; Browner, W.; Cauley, J.; Ensrud, K.; Genant, H.K.; Palermo, L.; Scott, J.; Vogt, T.M. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993, 341, 72–75. [Google Scholar]
- El Maghraoui, A.; Mouinga Abayi, D.A.; Ghozlani, I.; Mounach, A.; Nouijai, A.; Ghazi, M.; Mora, S.; Prinster, C.; Proverbio, M.C.; Bellini, A.; et al. Urinary markers of Bone turnover in healthy children and adolescents: Age-related changes and effect of puberty. Calcif. Tissue. Int. 1998, 63, 369–374. [Google Scholar]
- Reginster, J.Y.; Collette, J.; Neuprez, A.; Zegels, B.; Deroisy, R.; Bruyere, O. Role of biochemical markers of bone turnover as prognostic indicator of successful osteoporosis therapy. Bone 2008, 42, 832–836. [Google Scholar]
- Vasikaran, S.D.; Glendenning, P.; Morris, H.A. The role of biochemical markers of bone turnover in osteoporosis management in clinical practice. Clin. Biochem. Rev. 2006, 27, 119–121. [Google Scholar]
- Garnero, P. Biomarkers for osteoporosis management. Mol. Diag. Ther. 2008, 12, 157–170. [Google Scholar]
- Blumsohn, A.; Hannon, R.A.; Wrate, R.; Barton, J.; Al-Dehaimi, A.W.; Colwell, A.; Eastell, R. Biochemical markers of bone turnover in girls during puberty. Clin. Endocrinol. 1994, 40, 663–670. [Google Scholar]
- Iba, K.; Takada, J.; Hatakeyama, N.; Ozasa, Y.; Wada, T.; Yamashita, T. Changes in urinary NTX levels in patients with primary osteoporosis undergoing long-term bisphosphonate treatment. J. Orthop. Sci. 2008, 13, 438–441. [Google Scholar]
- Rosen, H.N.; Moses, A.C.; Garber, J.; Iloputaife, I.D.; Ross, D.S.; Lee, S.L.; Greenspan, S.L. Serum CTX: A new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif. Tissue. Int. 2000, 66, 100–103. [Google Scholar]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar]
- Qu, Q.; Zhu, Z.; Wang, Y.; Zhong, Z.; Zhao, J.; Qiao, F.; Du, X.; Wang, Z.; Yang, R.; Huang, L.; Yu, Y.; Zhou, L.; Chen, Z. Rapid and quantitative detection of Brucella by up-converting phosphor technology-based lateral-flow assay. J. Microbiol. Methods 2009, 79, 121–123. [Google Scholar]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar]
- Hanson, D.A.; Weis, M.A.; Bollen, A.M.; Maslan, S.L.; Singer, F.R.; Eyre, D.R. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J. Bone Miner. Res. 1992, 7, 1251–1258. [Google Scholar]
- Lee, M.; Chung, Y.C.; Lee, K.M.; Seong, W.K.; Kim, K.S.; Lee, S.D.; Park, M.S. Measurement of urinary N-telopeptides from type I collagen using a colloidal gold-based immunoassay. J. Biotechnol. 2011, 153, 176–180. [Google Scholar]
- Findlay, J.W.; Dillard, R.F. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 2007, 9, E260–E267. [Google Scholar]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar]
- Marx, R.E.; Cillo, J.E., Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral. Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar]
- Rosen, H.N.; Moses, A.C.; Garber, J.; Ross, D.S.; Lee, S.L.; Greenspan, S.L. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif. Tissue. Int. 1998, 63, 363–368. [Google Scholar]
- Baxter, I.; Rogers, A.; Eastell, R.; Peel, N. Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos. Int. 2012, in press. [Google Scholar]
- Bjarnason, N.H.; Henriksen, E.E.; Alexandersen, P.; Christgau, S.; Henriksen, D.B.; Christiansen, C. Mechanism of circadian variation in bone resorption. Bone 2002, 30, 307–313. [Google Scholar]

| N | 36 |
| Age (years) | 66.2 (SD 7.5) |
| Sex (M:F) | 4 : 32 |
| Measurements | |
| Serum CTx | 8.1 (3.1) ng/mL |
| Urine NTx | 121.6 (80.1) nmol BCE/mmol creatinine |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, K.M.; Lee, M.H.; Chung, C.Y.; Seong, W.K.; Lee, S.D.; Park, M.S. Measurement of Urinary N-Telopeptides and Serum C-Telopeptides from Type I Collagen Using a Lateral Flow-Based Immunoassay. Sensors 2013, 13, 165-174. https://doi.org/10.3390/s130100165
Lee KM, Lee MH, Chung CY, Seong WK, Lee SD, Park MS. Measurement of Urinary N-Telopeptides and Serum C-Telopeptides from Type I Collagen Using a Lateral Flow-Based Immunoassay. Sensors. 2013; 13(1):165-174. https://doi.org/10.3390/s130100165
Chicago/Turabian StyleLee, Kyoung Min, Min Ho Lee, Chin Youb Chung, Woo Kyeong Seong, Sang Dae Lee, and Moon Seok Park. 2013. "Measurement of Urinary N-Telopeptides and Serum C-Telopeptides from Type I Collagen Using a Lateral Flow-Based Immunoassay" Sensors 13, no. 1: 165-174. https://doi.org/10.3390/s130100165




