Monitoring Ion Activities In and Around Cells Using Ion-Selective Liquid-Membrane Microelectrodes
Abstract
:1. Introduction
- Numerous ISMs can be applied to a single cell at the same time, allowing numerous ion activities to be monitored simultaneously.
- ISMs can be applied to monitor ion activity at specific loci such as the cell surface or the cytoplasm.
- The reference electrode that is paired with the ISM for measurement of intracellular ion activity (see Section 5) provides a simultaneous measurement of membrane potential providing a more complete characterization of the transport processes that contribute to the changes in ion activities.
- In combination with vibrating probe technology [3], ISMs can be used to measure net ion fluxes.
- Ionophore-doped liquid membranes are imperfectly ion-selective.
- The use of ISMs to monitor intracellular ion activities is best applied to large cells that can be easily impaled with a microelectrode (e.g., Xenopus oocytes, which have a diameter that is greater than 1 mm; approximately 50–100 times larger than a typical mammalian cell).
2. Theory of ISMs
2.1. Gibbs Energy
2.2. Chemical Potential and Activity
2.3. Electrochemical Potential
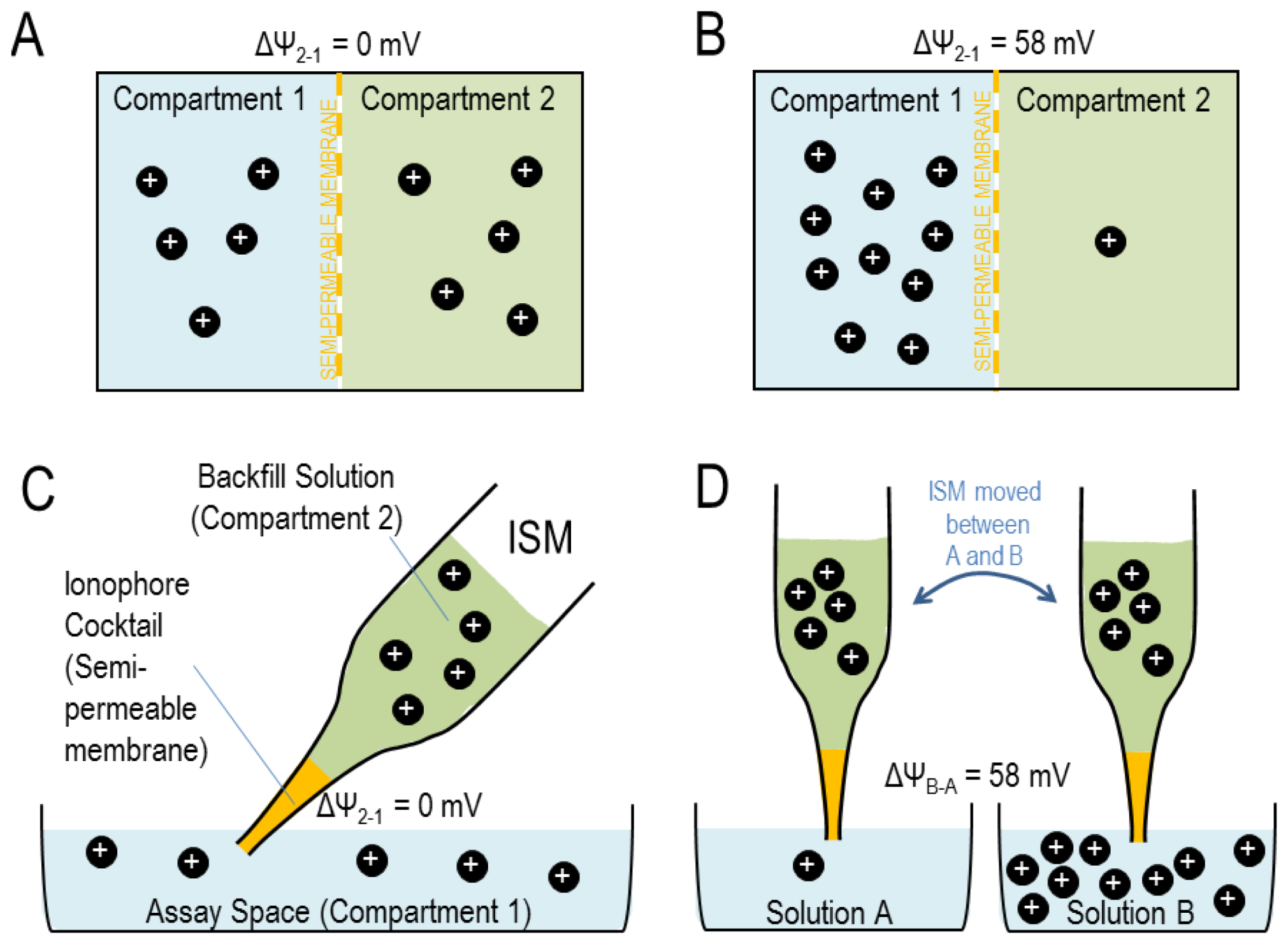
2.4. Electrochemical Potential Difference across a Semi-Permeable Membrane
2.5. Electrochemical Potential Difference across an Ion-Selective Liquid Membrane
3. The Composition of H+, Na+, K+, and Cl− Ionophore Cocktails and Backfill Solution
3.1. H+-Selective Ionophore Cocktails
3.2. Na+-Selective Ionophore Cocktails
3.3. K+-Selective Ionophore Cocktails
3.4. A Cl−-Selective Ionophore Cocktail
4. Fabricating ISMs
4.1. Pulling Glass Microelectrodes from Capillary Glass
4.2. Baking and Silanizing Empty Microelectrodes
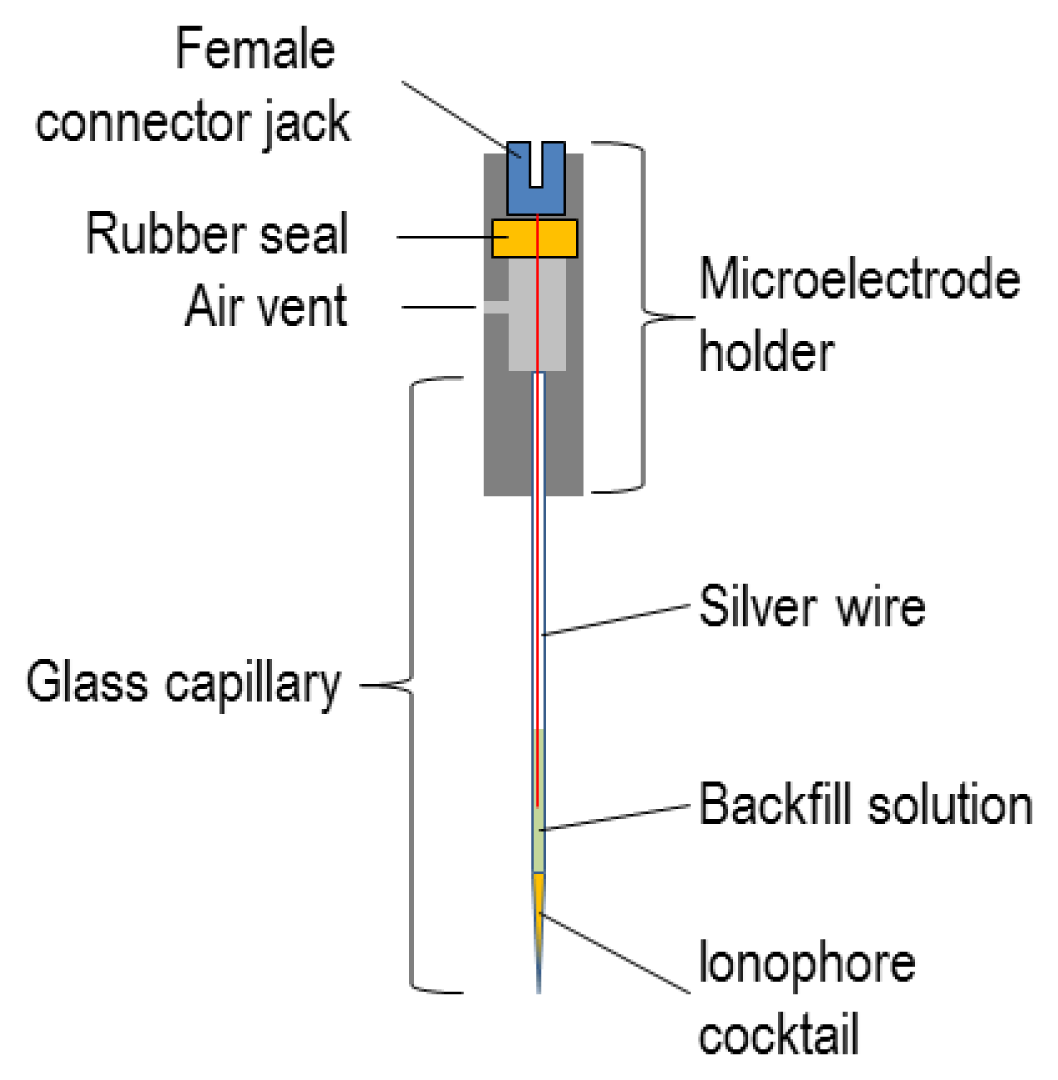
4.3. Filling and Backfilling the Microelectrodes
5. Use and Calibration of ISMs
5.1. Mounting ISMs
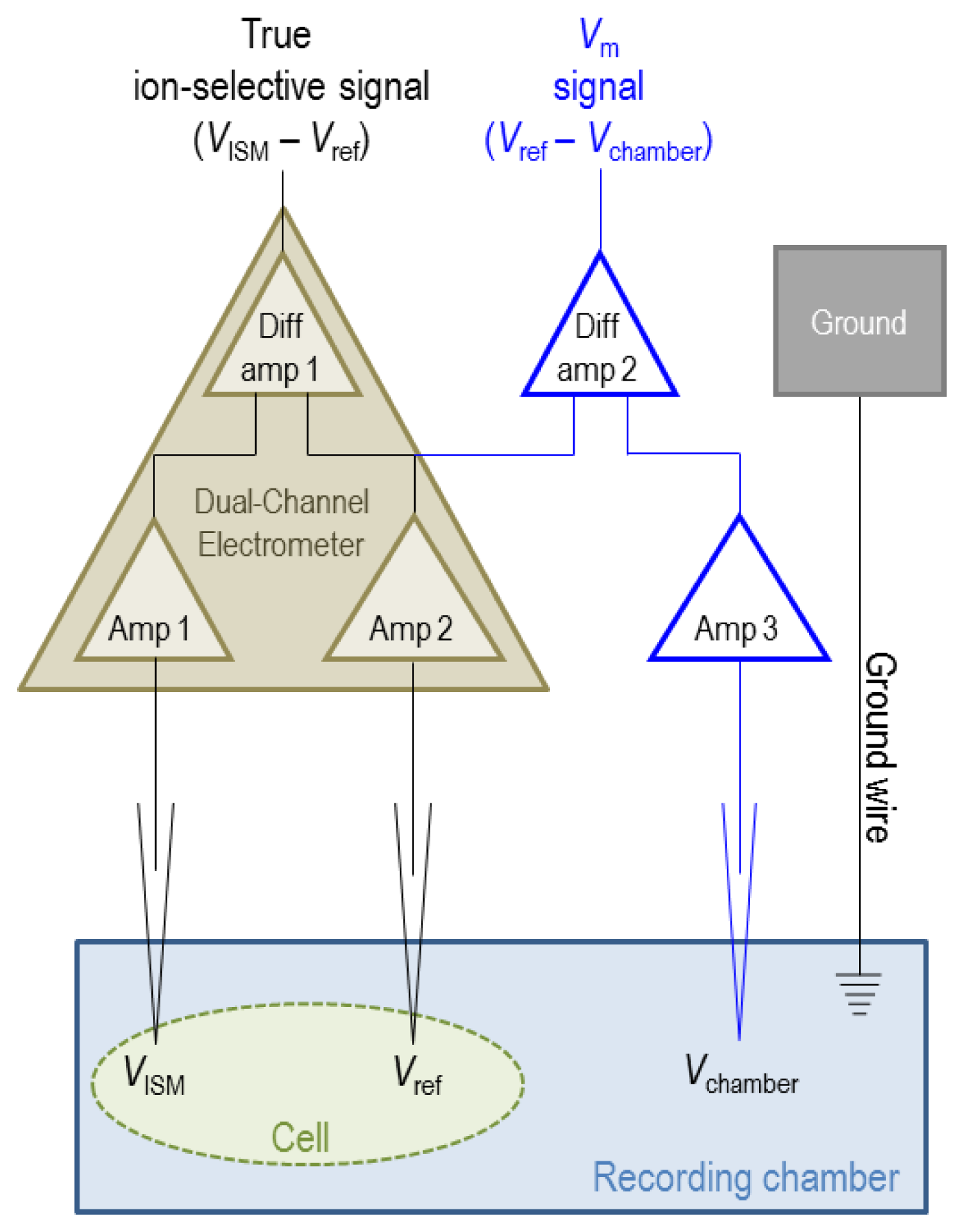
5.2. Electrical Set-Up
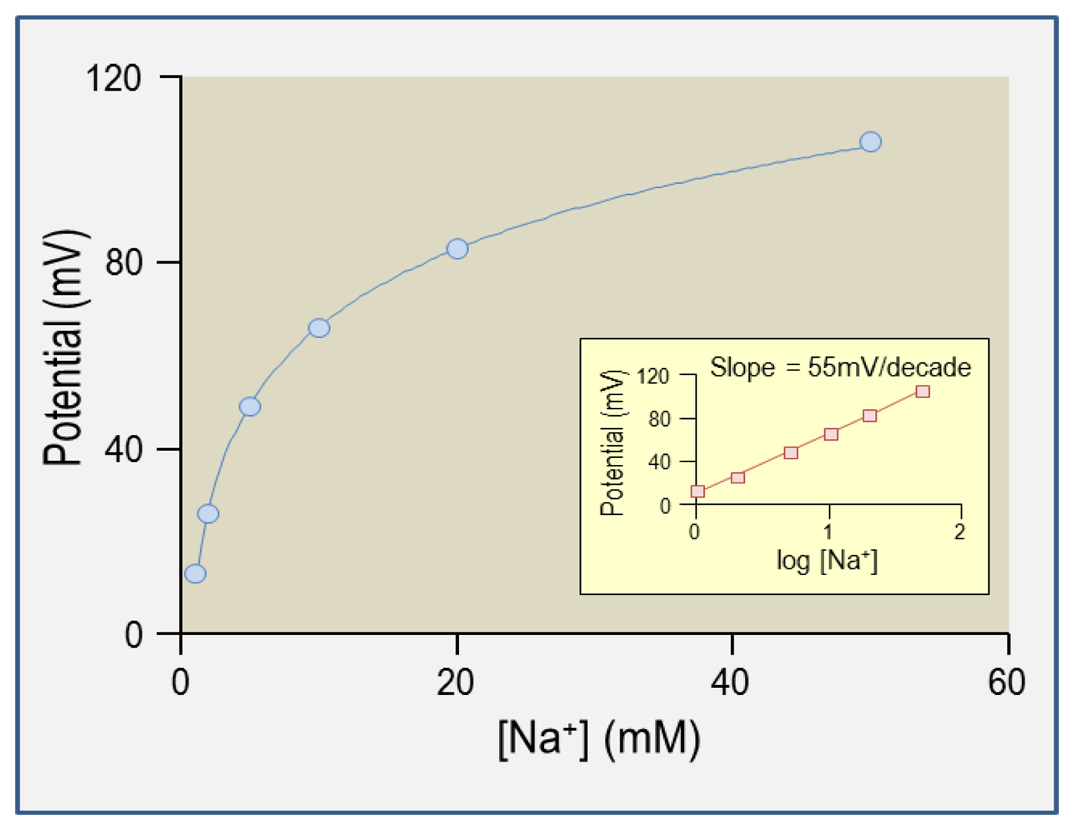
5.3. Calibration Procedure
6. Applications of ISMs
7. Outlook
Acknowledgments
References
- Markovich, D. Expression cloning and radiotracer uptakes in Xenopus laevis oocytes. Nat. Protoc. 2008, 3, 1975–1980. [Google Scholar]
- Tsien, R.Y. Fluorescent indicators of ion concentration. Methods Cell Biol. 1989, 30, 127–156. [Google Scholar]
- Reid, B.; Zhao, M. Ion-selective self-referencing probes for measuring specific ion flux. Commun Integr. Biol. 2011, 4, 524–527. [Google Scholar]
- Thomas, R.C. Ion-sensitive Intracellular Microelectrodes: How to Make and Use Them; Academic Press: London, UK, 1978. [Google Scholar]
- Voipio, J.; Pasternack, M. Ion-selective microelectrodes. In Microelectrode Techniques; Company of Biologists: Cambridge, UK, 1994; pp. 275–315. [Google Scholar]
- Thomas, R.C. Intracellular pH of snail neurones measured with a new pH-sensitive glass micro-electrode. J. Physiol. (Lond.) 1974, 238, 159–180. [Google Scholar]
- Boron, W.F.; Roos, A. Comparison of microelectrode DMO and methylamine methods for measuring intracellular pH. Am. J. Physiol. 1976, 231, 799–809. [Google Scholar]
- Boron, W.F.; De Weer, P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J. Gen. Physiol. 1976, 67, 91–112. [Google Scholar]
- Maccà, C. Response time of ion-selective electrodes: Current usage versus IUPAC recommendations. Anal. Chim. Acta. 2004, 512, 183–190. [Google Scholar]
- Ogden, D. Microelectrode techniques: the Plymouth Workshop handbook; Company of Biologists: Cambridge, UK, 1994. [Google Scholar]
- Koryta, J.; Štulík, K. Ion-Selective Electrodes; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Bührer, T.; Gehrig, P.; Simon, W. Neutral-carrier-based ion-selective microelectrodes design and application a review. Anal. Sci. 1988, 4, 547–557. [Google Scholar]
- Hinke, J.A. Thirty years of ion-selective microelectrodes: disappointments and successes. Can. J. Physiol. Pharmacol. 1987, 65, 873–878. [Google Scholar]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KpotA,B values. Pure Appl. Chem. 1995, 67, 507–518. [Google Scholar]
- Ammann, D.; Lanter, F.; Steiner, R.A.; Schulthess, P.; Shijo, Y.; Simon, W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal. Chem. 1981, 53, 2267–2269. [Google Scholar]
- Voipio, J.; Kaila, K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H+-sensitive microelectrode based on a PVC-gelled membrane. Pflügers Arch. 1993, 423, 193–201. [Google Scholar]
- Ammann, D.; Pretsch, E.; Simon, W.; Lindner, E.; Bezegh, A.; Pungor, E. Lipophilic salts as membrane additives and their influence on the properties of macro- and micro-electrodes based on neutral carriers. Anal. Chim. Acta. 1985, 171, 119–129. [Google Scholar]
- Steiner, R.A.; Oehme, M.; Ammann, D.; Simon, W. Neutral carrier sodium ion-selective microelectrode for intracellular studies. Anal. Chem. 1979, 51, 351–353. [Google Scholar]
- Ammann, D.; Anker, P. Neutral carrier sodium ion-selective microelectrode for extracellular studies. Neurosci. Lett. 1985, 57, 267–271. [Google Scholar]
- Hladky, S.B.; Leung, J.C.; Fitzgerald, W.J. The mechanism of ion conduction by valinomycin: analysis of charge pulse responses. Biophys. J. 1995, 69, 1758–1772. [Google Scholar]
- Neupert-Laves, K.; Dobler, M. The Crystal Structure of a K+ Complex of Valinomycin. Helvetica Chim. Acta. 1975, 58, 432–442. [Google Scholar]
- Oehme, M.; Simon, W. Microelectrode for potassium ions based on a neutral carrier and comparison of its characteristics with a cation exchanger sensor. Anal. Chim. Acta. 1976, 86, 21–25. [Google Scholar]
- Ammann, D.; Chao, P.S.; Simon, W. Valinomycin-based K+ selective microelectrodes with low electrical membrane resistance. Neurosci. Lett. 1987, 74, 221–226. [Google Scholar]
- Kondo, Y.; Buhrer, T.; Seiler, K.; Frömter, E.; Simon, W. A new double-barreled, ionophore-based microelectrode for chloride ions. Pflügers Arch. 1989, 414, 663–668. [Google Scholar]
- Parker, M.D.; Musa-Aziz, R.; Rojas, J.D.; Choi, I.; Daly, C.M.; Boron, W.F. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol. Chem. 2008, 283, 12777–12788. [Google Scholar]
- Chaniotakis, N.A.; Chasser, A.M.; Meyerhoff, M.E.; Groves, J.T. Influence of porphyrin structure on anion selectivities of manganese(III) porphyrin based membrane electrodes. Anal. Chem. 1988, 60, 185–188. [Google Scholar]
- Ammann, D.; Huser, M.; Kräutler, B.; Rusterholz, B.; Schulthess, P.; Lindemann, B.; Halder, E.; Simon, W. Anion Selectivity of Metalloporphyrins in Membranes. Helvetica Chim. Acta. 1986, 69, 849–854. [Google Scholar]
- Tripathi, S.; Morgunov, N.; Boulpaep, E.L. Submicron tip breakage and silanization control improve ion-selective microelectrodes. Am. J. Physiol. 1985, 249, C514–C521. [Google Scholar]
- Munoz, J.-L.; Deyhimi, F.; Coles, J.A. Silanization of glass in the making of ion-selective microelectrodes. J. Neurosci. Methods 1983, 8, 231–247. [Google Scholar]
- Chesler, M.; Chen, J.C.T.; Kraig, R.P. Determination of extracellular bicarbonate and carbon dioxide concentrations in brain slices using carbonate and pH-selective microelectrodes. J. Neurosci. Methods 1994, 53, 129–136. [Google Scholar]
- Smith, P.J. S.; Sanger, R.H.; Messerli, M.A. Principles, development and applications of self-referencing electrochemical microelectrodes to the determination of fluxes at cell membranes. In Electrochemical Methods for Neuroscience; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cicirelli, M.F.; Robinson, K.R.; Smith, L.D. Internal pH of Xenopus oocytes: a study of the mechanism and role of pH changes during meiotic maturation. Developmental. Biol. 1983, 100, 133–146. [Google Scholar]
- Bröer, S.; Schneider, H.P.; Bröer, A.; Rahman, B.; Hamprecht, B.; Deitmer, J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174. [Google Scholar]
- Fei, Y.J.; Kanai, Y.; Nussberger, S.; Ganapathy, V.; Leibach, F.H.; Romero, M.F.; Singh, S.K.; Boron, W.F.; Hediger, M.A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 1994, 368, 563–566. [Google Scholar]
- Romero, M.F.; Hediger, M.A.; Boulpaep, E.L.; Boron, W.F. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 1997, 387, 409–413. [Google Scholar]
- Nakhoul, N.L.; Davis, B.A.; Romero, M.F.; Boron, W.F. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. 1998, 274, C543–C548. [Google Scholar]
- Musa-Aziz, R.; Jiang, L.; Chen, L.M.; Behar, K.L.; Boron, W.F. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J. Membr. Biol. 2009, 228, 15–31. [Google Scholar]
- Dinour, D.; Chang, M.H.; Satoh, J.; Smith, B.L.; Angle, N.; Knecht, A.; Serban, I.; Holtzman, E.J.; Romero, M.F. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J. Biol. Chem. 2004, 279, 52238–52246. [Google Scholar]
- Parker, M.D.; Qin, X.; Williamson, R.C.; Toye, A.M.; Boron, W.F. HCO3−-independent conductance with a mutant Na+/HCO3− cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalemic paralysis. J. Physiol. 2012, 590, 2009–2034. [Google Scholar]
- Suzuki, M.; Vaisbich, M.H.; Yamada, H.; Horita, S.; Li, Y.; Sekine, T.; Moriyama, N.; Igarashi, T.; Endo, Y.; Cardoso, T.P.; De Sa, L.C.; Koch, V.H.; Seki, G.; Fujita, T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflügers Arch. 2008, 455, 583–593. [Google Scholar]
- Garber, S.S.; Messerli, M.A.; Hubert, M.; Lewis, R.; Hammar, K.; Indyk, E.; Smith, P.J.S. Monitoring Cl- movement in single cells exposed to hypotonic solution. J. Membr. Biol. 2005, 203, 101–110. [Google Scholar]
- Grichtchenko, I.I.; Chesler, M. Depolarization-induced acid secretion in gliotic hippocampal slices. Neuroscience 1994, 62, 1057–1070. [Google Scholar]
- Grichtchenko, I.I.; Chesler, M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience 1994, 62, 1071–1078. [Google Scholar]
- Kraig, R.P.; Ferreira-Filho, C.R.; Nicholson, C. Alkaline and acid transients in cerebellar microenvironment. J. Neurophysiol. 1983, 49, 831–850. [Google Scholar]
- Rodríguez, E.C.; Robertson, R.M. Protective effect of hypothermia on brain potassium homeostasis during repetitive anoxia in Drosophila melanogaster. J. Exp. Biol. 2012, 215, 4157–4165. [Google Scholar]
- Sasaki, S.; Shiigai, T.; Takeuchi, J. Intracellular pH in the isolated perfused rabbit proximal tubule. Am. J. Physiol. 1985, 249, F417–F423. [Google Scholar]
- Biagi, B.A.; Vance, B.A. Microelectrode characterization of the basolateral membrane of rabbit S3 proximal tubule. J. Membrane Biol. 1989, 108, 53–60. [Google Scholar]
- Semb, S.O.; Amundsen, B.; Sejersted, O.M. A new improved way of making double-barrelled ion-selective micro-electrodes. Acta Physiol. Scand. 1997, 161, 1–5. [Google Scholar]
- Ianowski, J.P.; Christensen, R.J.; O'Donnell, M.J. Intracellular ion activities in Malpighian tubule cells of Rhodnius prolixus: Evaluation of Na+-K+-2Cl- cotransport across the basolateral membrane. J. Exp. Biol. 2002, 205, 1645–1655. [Google Scholar]
- Blanchard, M.G.; Longpre, J.P.; Wallendorff, B.; Lapointe, J.Y. Measuring ion transport activities in Xenopus oocytes using the ion-trap technique. Am. J. Physiol. Cell Physiol. 2008, 295, C1464–C1472. [Google Scholar]
- Parker, M.D.; Musa-Aziz, R.; Boron, W.F. Letter to the editor: The use of extracellular, ion-selective microelectrodes to study the function of heterologously expressed transporters in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 2009, 296, C1243. [Google Scholar]
- Messerli, M.A.; Collis, L.P.; Smith, P.J. S. Ion trapping with fast-response ion-selective microelectrodes enhances detection of extracellular ion channel gradients. Biophys. J. 2009, 96, 1597–1605. [Google Scholar]
- Musa-Aziz, R.; Chen, L.; Pelletier, M.F.; Boron, W.F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Nat. Acad. Sci. USA 2009, 106, 5406–5411. [Google Scholar]
- Longpré, J.-P.; Lapointe, J.-Y. Determination of the Na+/glucose cotransporter (SGLT1) turnover rate using the ion-trap technique. Biophys. J. 2011, 100, 52–59. [Google Scholar]
- Cotton, C.U.; Weinstein, A.M.; Reuss, L. Osmotic water permeability of Necturus gallbladder epithelium. J. Gen. Physiol. 1989, 93, 649–679. [Google Scholar]
- Chang, M.H.; Plata, C.; Kurita, Y.; Kato, A.; Hirose, S.; Romero, M.F. Euryhaline Pufferfish NBCe1 differs from non-marine species NBCe1 physiology. Am. J. Physiol. Cell Physiol. 2011, 302, C1083–C1095. [Google Scholar]
- Johnson, R.D.; Gavalas, V.G.; Daunert, S.; Bachas, L.G. Microfluidic ion-sensing devices. Anal. Chim. Acta. 2008, 613, 20–30. [Google Scholar]
- Jayakannan, M.; Babourina, O.; Rengel, Z. Improved measurements of Na+ fluxes in plants using calixarene-based microelectrodes. J. Plant Phys. 2011, 168, 1045–1051. [Google Scholar]
- Kim, D.W.; Park, K.-W.; Yang, M.-H.; Kim, T.H.; Mahajan, R.K.; Kim, J.S. Selective uranyl ion detection by polymeric ion-selective electrodes based on salphenH2 derivatives. Talanta 2007, 74, 223–228. [Google Scholar]
- Moriuchi-Kawakami, T.; Tokunaga, Y.; Yamamoto, H.; Shibutani, Y. Ion-selective electrodes based on L-tryptophan and L-tyrosine. Talanta 2012, 94, 99–103. [Google Scholar]
- Faridbod, F.; Norouzi, P.; Dinarvand, R.; Ganjali, M.R. Developments in the Field of conducting and non-conducting polymer based potentiometric membrane sensors for ions over the past decade. Sensors 2008, 8, 2331–2412. [Google Scholar]

| Component | Composition | Requisite Characteristics | |
|---|---|---|---|
| Cocktail | Ionophore | 10% (w/w) tridodecylamine (TDDA; CAS no. 102-87-4) | TDDA is a lipophilic amine that is predominantly uncharged in an organic solution equilibrated with a neutral aqueous solution, making it a neutral proton carrier [16]. |
| Solvent | 89.3% 2-nitrophenyl octyl ether (o-NPOE; CAS no. 37682-29-4) | ||
| Additive | 0.7% potassium tetrakis(4-chlorophenyl) borate (KTCPB; CAS no. 14680-77-4) | Reduces anion interference and electrical resistance without compromising ion-selectivity [16,18]. | |
| Backfill | 40 mM KH2PO4, 15 mM NaCl, pH 7.0 with 23 mM NaOH [16] | Buffered electrolyte solution | |
| Component | Composition | Requisite Characteristics | |
|---|---|---|---|
| Cocktail | Ionophore | 10% (w/w) N,N′,N″-Triheptyl-N,N′,N″-trimethyl- 4,4′,4″-propylidynetris(3-oxa- butyramide) (CAS no. 61183-76-4) | Forms a structure with a Na+ co-ordinating site that is relatively selective over intracellular interfering ions in the intracellular space (e.g., K+). |
| Solvent | 89.5% o-NPOE | ||
| Additive | 0.5% sodium tetraphenyl borate (NaTPB; CAS no. 143-66-8) | Reduces anion interference, and electrical resistance without compromising ion-selectivity [18,19]. | |
| Backfill | 10 mM NaCl | Contains no interfering ions. | |
| Component | Composition | Requisite Characteristics | |
|---|---|---|---|
| Cocktail | Ionophore | 5% (w/w) valinomycin (CAS no. 2001-95-8) | Valinomycin forms a ring structure that selectively co-ordinates K+[22]. |
| Solvents | 25% 1,2-dimethyl-3-nitrobenzene (CAS no. 83-41-0) | ||
| 68% dibutyl sebacate (CAS no. 109-43-3) | |||
| Additive | 2% KTCPB | Contributes to cation-sensing, reducing anion-interference and reduces electrical resistance without compromising ion-selectivity [16,18]. | |
| Backfill | 10–100 mM KCl [23,24] | Contains no interfering ions. | |
| Component | Composition | Requisite Characteristics | |
|---|---|---|---|
| Cocktail | Ionophore | 5% (w/w) m-Tetraphenyl-porphyrin manganese(III)-chloride complex (CAS no. 32195-55-4) | Ring structure that co-ordinates Mn3+, which has a greater affinity for Cl− than for HCO3−, the other major physiological anion, conferring a useful selectivity to the cocktail [25,27,28]. |
| Solvents | 90% o-NPOE | The addition of decanol reduces the electrical resistance of the cocktail, and increases its selectivity but at the cost of a reduced response time [25]. | |
| 4% decanol (CAS no. 112-30-1) | |||
| Additive | 1% Tetradodecylammonium tetrakis(4-chlorophenyl)-borate (CAS no. 100582-42-8) | Reduces electrical resistance without compromising ion-selectivity. | |
| Backfill | 100 mM NaCl buffered with 10 mM Tris, pH 7.4 with H2SO4 [25]. | Buffered Cl-containing solution that lacks interfering anions (divalent anions do not substantially interfere with porphyrin-based ionophores). | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, S.-K.; Boron, W.F.; Parker, M.D. Monitoring Ion Activities In and Around Cells Using Ion-Selective Liquid-Membrane Microelectrodes. Sensors 2013, 13, 984-1003. https://doi.org/10.3390/s130100984
Lee S-K, Boron WF, Parker MD. Monitoring Ion Activities In and Around Cells Using Ion-Selective Liquid-Membrane Microelectrodes. Sensors. 2013; 13(1):984-1003. https://doi.org/10.3390/s130100984
Chicago/Turabian StyleLee, Seong-Ki, Walter F. Boron, and Mark D. Parker. 2013. "Monitoring Ion Activities In and Around Cells Using Ion-Selective Liquid-Membrane Microelectrodes" Sensors 13, no. 1: 984-1003. https://doi.org/10.3390/s130100984




