A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity
Abstract
: A new fluorescent Zn2+ chemosensor (P1) based on a functionalized porphyrin was synthesized and characterized. P1 displayed dramatic ratiometric variations in absorption and fluorescent emission spectra upon exposure to Zn2+ due to the formation of a 1:1 Zn2+/P1 complex. The sensor also exhibited high selectivity and sensitivity toward Zn2+ over other common metal ions in the physiological pH range with a detection limit of 1.8 μM. The sensor showed fast response times and excellent reproducibility, thus confirming its potential applicability as a fluorescent sensor for Zn2+ sensing.1. Introduction
Zinc ions (Zn2+), the second most abundant transition-metal ions in the human body, play diverse roles in biological processes, such as gene expression, metalloenzyme function, and neurotransmission [1–3]. Though zinc is a relatively nontoxic element, it can be toxic if consumed in large enough quantities. For example, zinc is a metal pollutant of environment, significant concentrations of which may reduce the soil microbial activity causing phytotoxic effects and it is a common contaminant in agricultural and food wastes. Consequently, the exploitation of chemosensors with high selectivity and sensitivity for detecting trace amounts of Zn2+ has attracted increased research interest. Given that Zn2+ does not produce spectroscopic or magnetic signals because of its 3d104s0 electronic configuration, the fluorescence method is the primary method of choice for Zn2+ determination [4,5]. Several studies have reported the successful application of fluorescent sensors based on quinoline [6,7], fluorescein [8,9], coumarin [10], indole [11], naphthalimide [12], and peptides [13] in the detection of Zn2+. However, the development of new small molecular fluorescent sensors with extremely high sensitivity for Zn2+ and high selectivity over other relevant metal ions remains desirable.
Among various fluorescent sensors currently available, porphyrin and its derivatives are the first class of probes to be developed for Zn2+. These organic compounds exhibit good photostability, high absorption coefficients in the region from 400 nm to 450 nm (visible range), large Stokes shifts that minimize the effects of background fluorescence, and appreciable changes in spectral shift upon ligand binding [14–17]. Ratiometric fluorescence measurements have recently been conducted to detect metal ions. The method involves the observation of changes in the ratio of emissions at two wavelengths, thereby increasing the selectivity and sensitivity of determination and eliminating most or all of the possible variabilities arising from differences in instrumental efficiency and environmental effects [18–24]. In this study, we report a novel ratiometric fluorescent probe P1 for Zn2+ based on a porphyrin derivative (see Scheme 1). To achieve the requirement of high selectivity toward Zn2+, a strong chelator bearing three nitrogen atoms was incorporated into the porphyrin. These nitrogen atoms can form a cavity that can suitably contain Zn2+. The amine group of the chelator is a hydrophilic group, which can enhance the water solubility of the sensor [25].
2. Experimental Section
2.1. Materials and Apparatus
All chemicals were purchased from commercial suppliers. Methanol, ethanol, and acetonitrile were dried and distilled before use. N,N′-dimethylformamide (DMF) of analytical reagent grade was dried over 4 Å molecular sieves. All other chemicals were of analytical reagent grade and used without further purification, unless otherwise specified. 5-(4-Bromophenyl)-10,15,20-triphenylporphyrin (BrTPP) was synthesized using a reported procedure [26]. The 1H-NMR spectra were obtained on a Bruker (Advance DMX500) NMR spectrometer. Mass spectra (MS) were recorded with a Bruker Esquire 3000 Plus spectrophotometer (Bruker-Franzen Analytik GmbH, Bremen, Germany). IR spectra were recorded on a spectrometer (Nicolet, Nexus-470, Madison, WI, USA) for the compounds in the solid state, prepared as KBr discs. Ultraviolet visible (UV−vis) absorption spectra were obtained using a Shimadzu UV 2450 spectrophotometer (Shimadzu, Kyoto, Japan). Fluorescence spectra measurements were performed on a Shimadzu RF-5301 PC (Shimadzu) fluorescence spectrophotometer. Both excitation and emission slits measured 3 nm. All pH measurements were made with a pH-10C digital pH meter. All titration experiments were run twice to generate reliable data.
2.2. Synthesis of P1
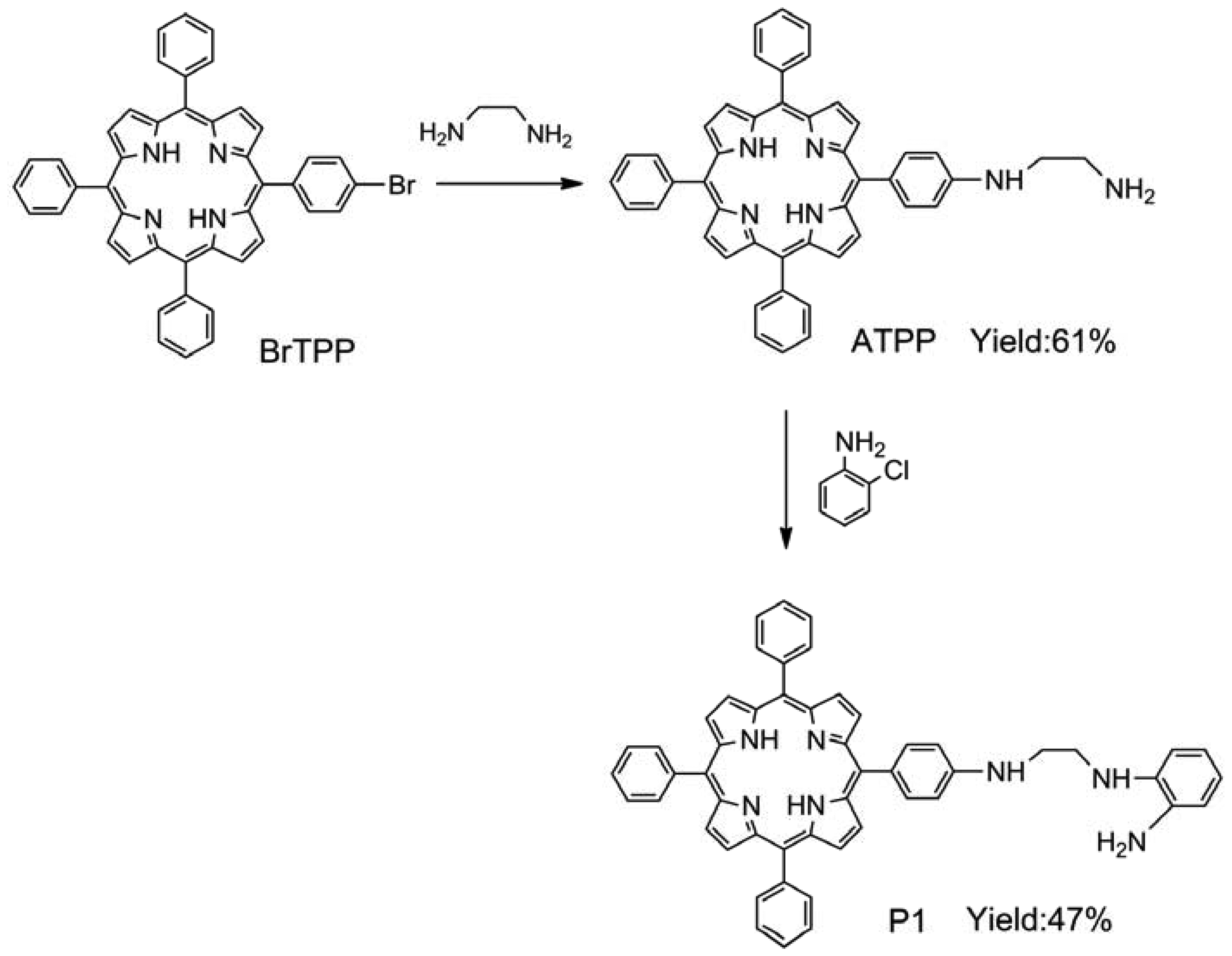
BrTPP (2.08 g, 3 mmol) and ethylenediamine (6.0 mL, 90 mmol) were dissolved in DMF (15 mL). Then CuSO4·5H2O (0.1 g, 0.4 mmol) was added to this solution. The resulting mixture was heated under reflux for 5 h. After cooling to room temperature, the solution was poured into water (100 mL). The precipitate was collected by filtration, washed with water, and dried to yield a purple powder. The product was purified by silica gel column chromatography using dichloromethane/methanol (15:1, v/v) as an eluent to afford 5-[4-(aminoethylene)amino]-10,15,20-triphenylporphyrin (ATPP). Yield: 61%. 1H-NMR (CDCl3, δ): 8.81 (s, 4H), 8.65 (s, 4H), 8.29 (m, 3H), 7.67–7.80 (m, 12H), 7.61–7.67 (m, 4H), 6.98 (m, 1H), 4.12 (s, 2H), 3.59 (m, 2H), 2.68 (m, 2H), −2.76 (s, 2H); MS (ESI): 673.2 [M + 1] +; IR (KBr, ν) 3,306, 3,267, 3,227, 3,005, 2,926, 1,080, 899 cm−1 (see Figures S1–S3 in Supplementary Material). For P1 synthesis, ATPP (0.404 g, 0.6 mmol), ortho-aminophenol (0.055 g, 0.5 mmol), and potassium iodide (0.008 g, 0.05 mmol) were added to an acetonitrile solution (30 mL). The reaction mixture was stirred and heated by refluxing for 6 h under a nitrogen atmosphere. After removal of the solvent, the crude mixture was purified by silica gel column chromatography using dichloromethane/methanol (12:1, v/v) as the eluent, thus yielding compound P1. Yield: 47%. 1H-NMR (CDCl3, δ): 8.83 (s, 4H), 8.70 (s, 4H), 8.31 (m, 3H), 7.61–7.71 (m, 12H), 7.57–7.59 (m, 4H), 7.06 (s, 2H), 6.74–6.92 (m, 4H), 5.32 (s, 2H), 3.94 (m, 4H), −2.81 (s, 2H); MS (ESI): 764.3 [M + 1] +; IR (KBr, ν) 3,452, 3,417, 3,356, 3,030, 2,912, 1,489, 867 cm−1 (see Figures S4–S6 in Supplementary Material).
2.3. Measurement Procedures
Stock solutions of 20 μM P1 and 1.0 × 104 μM Zn2+ were prepared by dissolving P1 in absolute ethanol and ZnSO4·7H2O in doubly distilled water, respectively. The latter solution was further diluted stepwise to yield working solutions with concentrations ranging from 1.0 × 103 μM to 1.0 μM. The wide-pH range solutions were prepared by adjusting 5.0 × 104 μM Tris–HCl solution with HCl or NaOH solution. Solutions of other metal ions were prepared by dissolving NaCl, KCl, CaCl2, Cd(NO3)2·4H2O, Cr(NO3)3·9H2O, Co(NO3)2·6H2O, MgSO4, Cu(NO3)2·3H2O, Pb(NO3)2, MnSO4·H2O, FeSO4·7H2O and HgCl2 in doubly distilled water (1.0 × 103 μM).
A complex solution of Zn2+/P1 was prepared by adding 5.0 mL of the stock solution of P1 and 1.0 mL of the stock solution of Zn2+ to a 10.0 mL volumetric flask. The mixture was then diluted to 10.0 mL with Tris–HCl buffer solution (pH 7.4). The final concentrations of P1 and Zn2+ in the complex solutions were 10 μM and 100 μM–0.1 μM, respectively. The solution was protected from light and kept at 4 °C until use. A blank solution of P1 was prepared under the same conditions without Zn2+.
3. Results and Discussion
3.1. Preparation and Characterization of P1
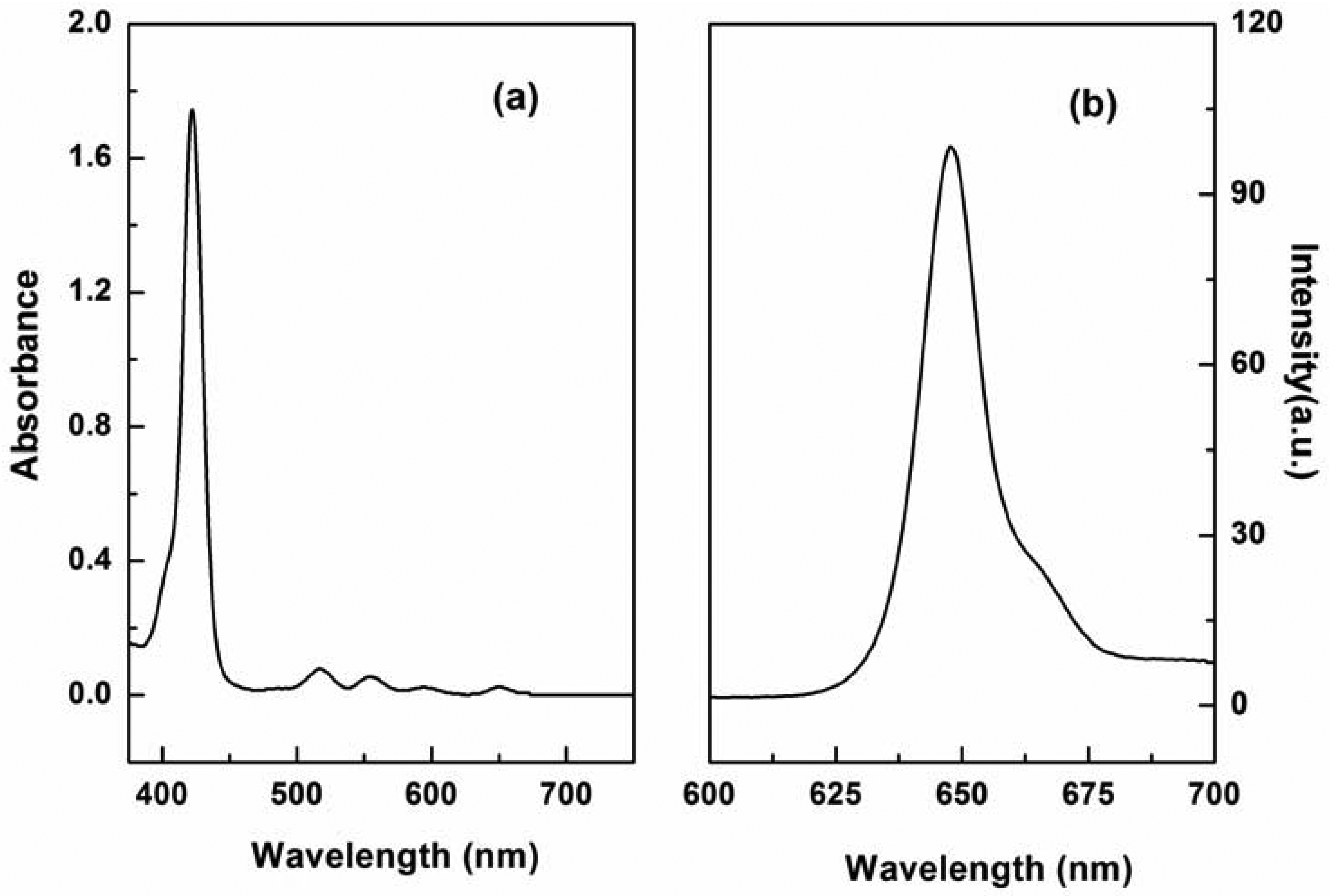
P1 was easily synthesized with 47% yield by a two-step procedure [27,28]. The absorption spectrum of P1 displayed the characteristic transitions of porphyrin with an intense Soret band at 422 nm and four weak Q-bands at 519, 555, 594, and 650 nm (see Figure 1(a)). This result is in agreement with our finding that P1 exhibits a typical fluorescence peak at 650 nm when excited at 420 nm (see Figure 1(b)). Tetraphenylporphyrin was used as a standard (Φref = 0.15) [29], and the fluorescence quantum yield (ΦF) of P1 in N,N′-dimethylacetamide was determined to be 0.20, according to the method described by Demas and Crosby [30].
3.2. Sensing Properties of P1 to Zn2+
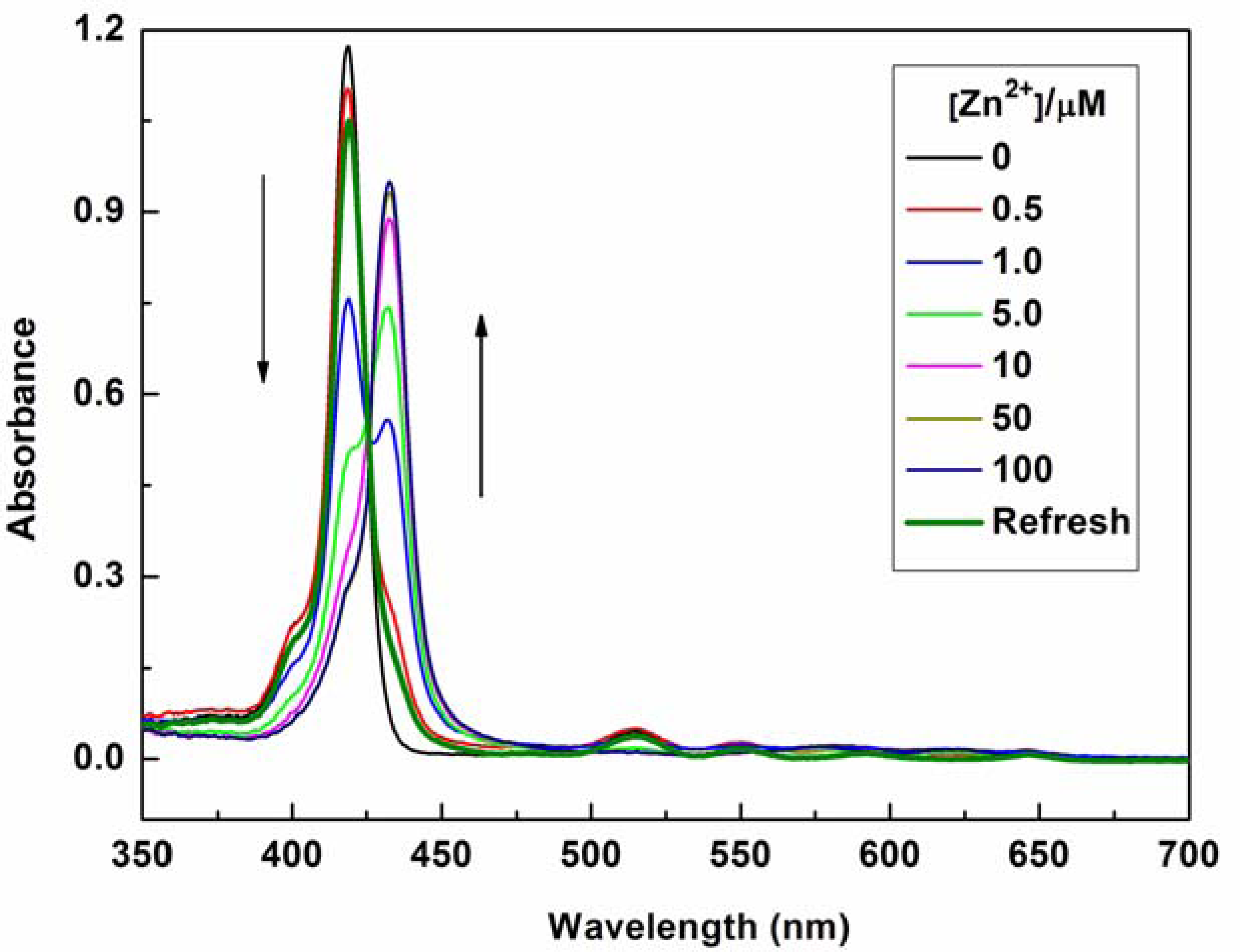
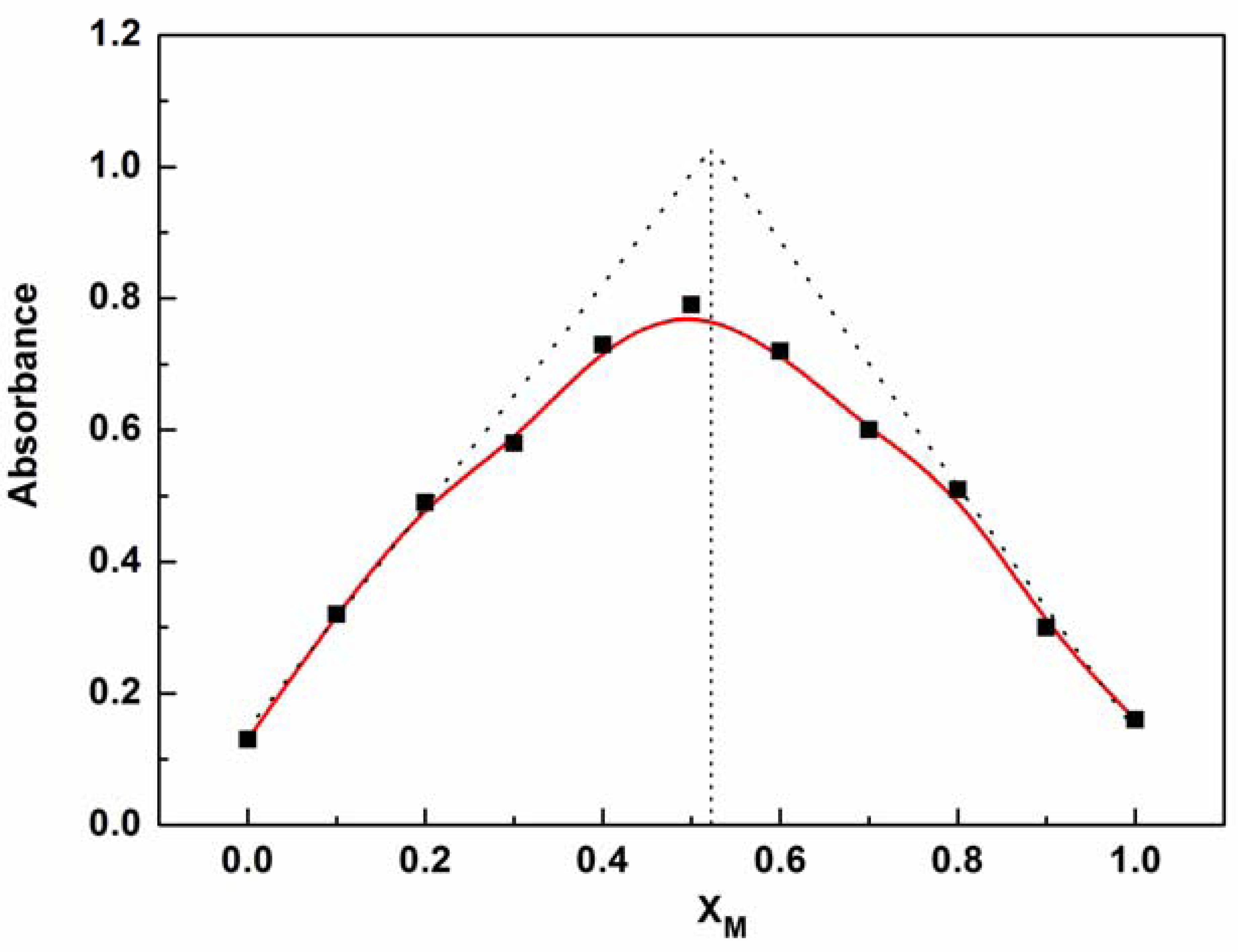
The mode of coordination of P1 with Zn2+ was investigated by spectrophotometric titration. Upon the gradual addition of Zn2+ (0.0–10.0 equiv.) to P1 (10 μM) solution, the Soret band of P1 showed a gradual bathochromic shift from 422 nm to 436 nm with an isosbestic point at 426 nm and a decrease in the Qy(0–0) band at 519 nm (see Figure 2). This result indicates that a new species is only produced during the titration [21–24,31–36]. The changes in the UV–vis spectrum of the solutions can be interpreted as the electron rearrangement in P1 through strong Zn2+ coordination. To further understand the binding behavior and determine the stoichiometry of the formed complex, the Job's plot for the absorbance was determined by keeping the sum of initial concentrations of Zn2+ and P1 constant at 10 μM and changing the molar ratio of Zn2+ (XM = ([Zn2+]/([Zn2+] + [P1])) from 0 to 1. As shown in Figure 3, a plot of absorbance at 436 nm versus XM shows that the absorbance value is highest at a molar fraction of ca. 0.5, indicating that the complex formed between P1 and Zn2+ follows a 1:1 stoichiometry [37,38].
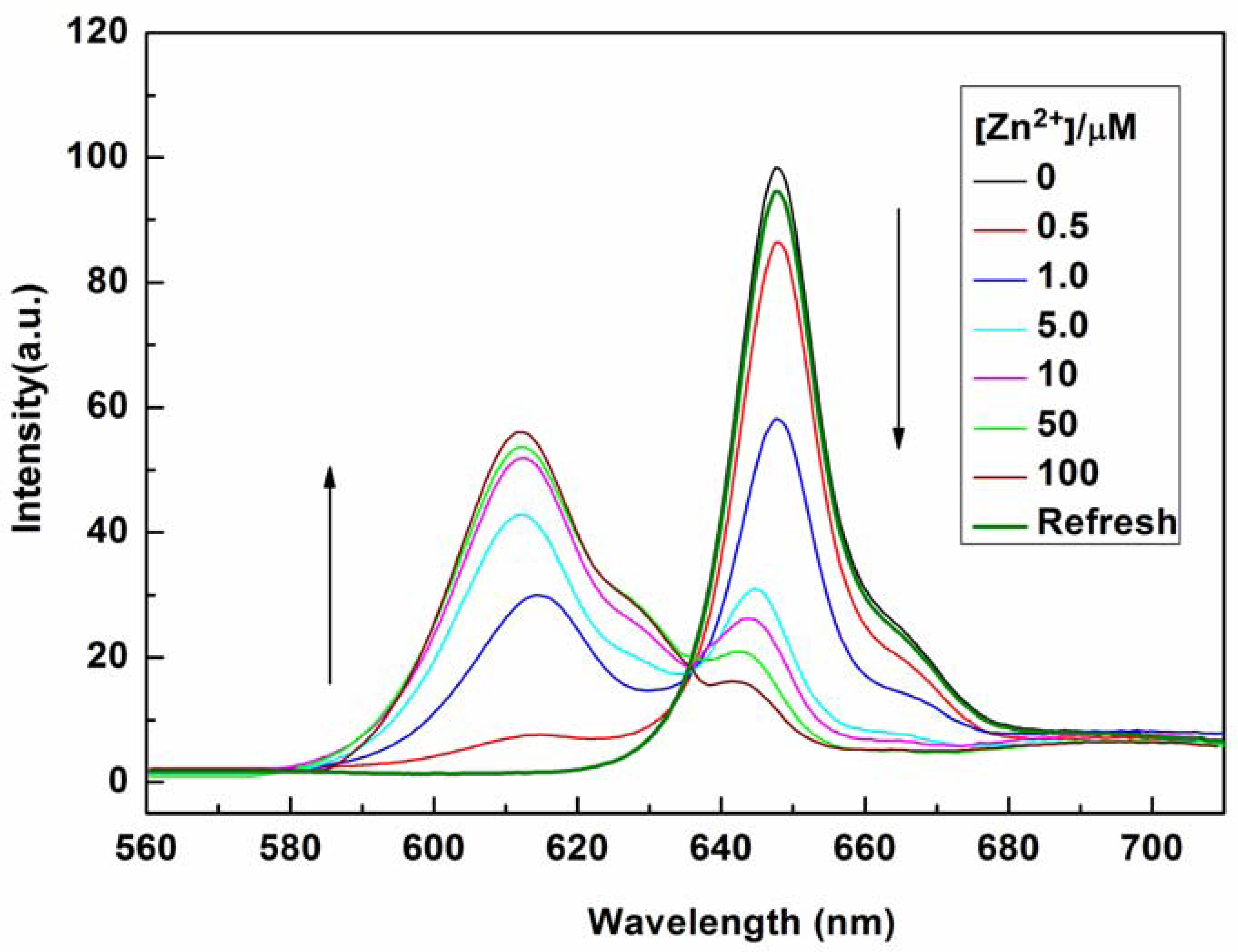
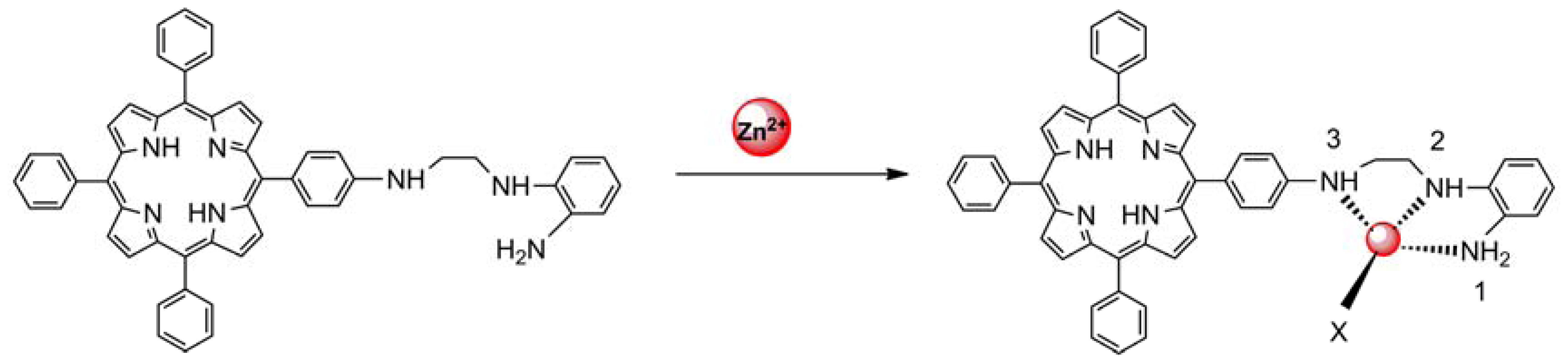
The sensing properties of P1 towards Zn2+ were also investigated by emission spectral titration. Figure 4 reveals that P1 could be developed as a ratiometric sensor for Zn2+. Upon the gradual addition of Zn2+ (0.0–10.0 equiv.) to a P1 solution (10 μM), the emission peak of P1 at 650 nm decreased with the concomitant formation of new peak at 610 nm. When Zn2+ ions were further added to the P1 solution, the ratiometric change in fluorescence spectra became evident with a clear isoemission point at 636 nm. The Zn2+-induced emission shift of P1 also confirmed the formation of a Zn2+/P1 complex. To investigate the binding behavior of P1 with Zn2+, 1H-NMR spectra of P1 in the absence and presence of Zn2+ were recorded at room temperature as shown in Figure S7. Upon addition of 1.0 equiv of Zn2+, the protons signals for the H2, H3 and NH2 groups underwent distinct downfield shifts due to the deshielding effect of the metal ion, implying that the three N atoms (labeled N1, N2, and N3 in Scheme 2) should be involved directly in Zn2+ coordination. In addition, the coordination of Zn2+ by the N2, N3 atoms promoted a downfield shift for all aromatic protons (H4-H7) in the phenyl ring of the ligating group. Thus, we can deduce a possible coordination mode between Zn2+ and P1, as shown in Scheme 2. The three N atoms (labeled N1, N2, and N3 in Scheme 2) participate in the coordination with Zn2+. The fourth ligand (X) was considered to be SO42− by consideration of the charge balance in the solution [3,39]. Plotting the concentrations of Zn2+ against the variation in absorbance (ΔA), which refers to the difference in the absorbance intensities of P1 at 436 nm before and after exposure to various concentrations of Zn2+, yields the following equation [40]:
We investigated the time evolution of the response of P1 to 2.0 equiv. of Zn2+ in EtOH/H2O solution (1:1, v/v) (see Figure S8 in Supplementary Material). The interaction of P1 with Zn2+ was completed in less than 2 min. Therefore, this system could be used for real-time tracking of Zn2+.
The optical responses of P1 to Zn2+ were found to be fully reversible. When ethylene diamine tetraacetic acid (EDTA) was subsequently added to the complexed solution of P1 and Zn2+, an absorption peak at 422 nm and a fluorescence signal at 650 nm were completely recovered. This result demonstrates that the binding between P1 and Zn2+ is chemically reversible and not a cation-catalyzed reaction (see Figures 2 and 4).
3.3. Effect of pH
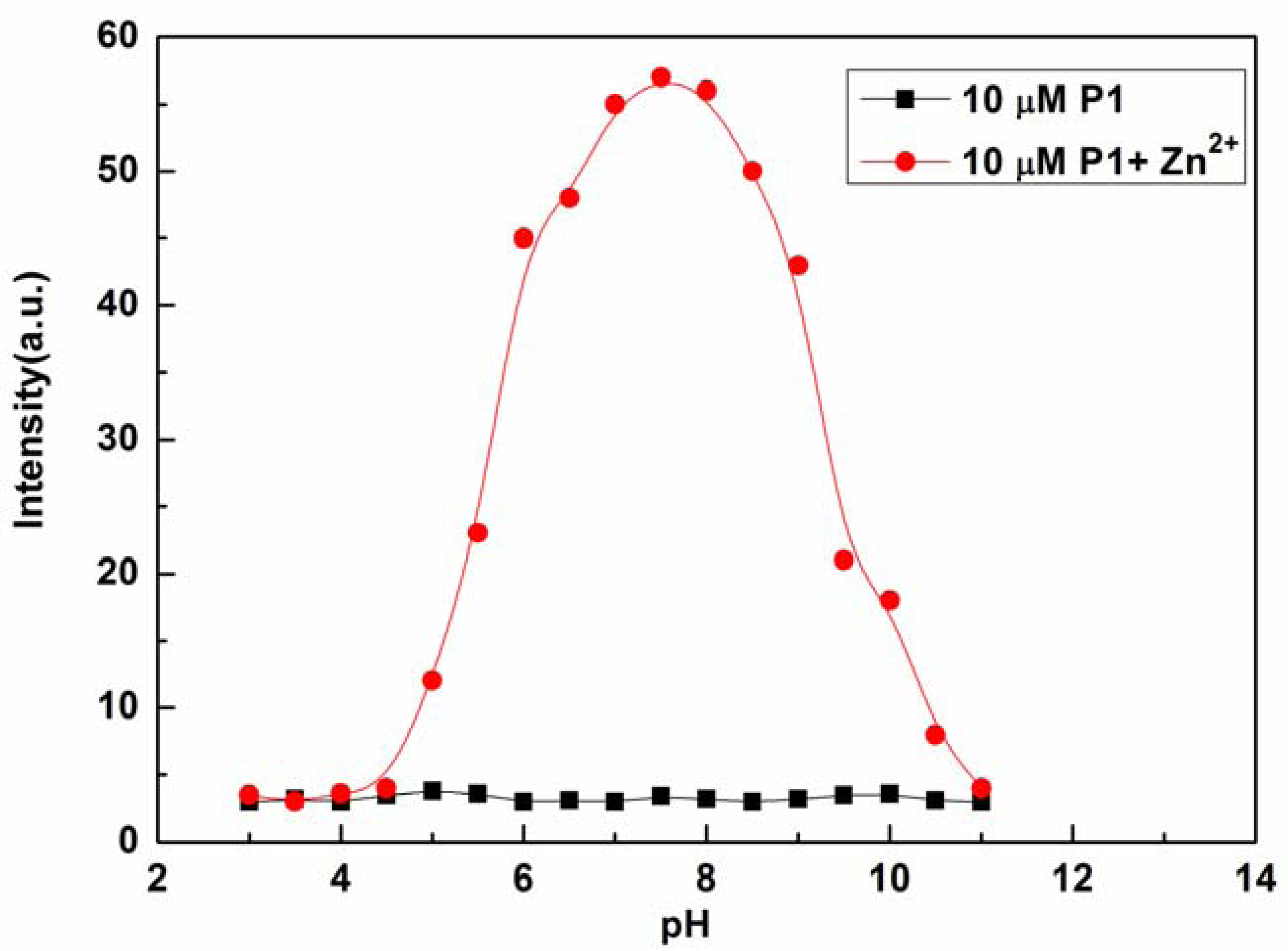
The effects of pH on the fluorescence intensity of P1 at 610 nm in the presence of Zn2+ were determined in the pH range from 3.0 to 11.0 with the concentration of Zn2+ fixed at 10 μM (see Figure 5). The fluorescence intensity of P1 at 610 nm decreased with decreasing pH value, which may be caused by the protonation of P1. On the other hand, high pH values led to the precipitation of Zn(OH)2, which, in turn, reduced complexation with P1. At pH values ranging from 6.5 to 8.5, acidity did not appear to affect the determination of Zn2+ with P1 [41]. Therefore, the response behavior of P1 is pH independent within the relevant biological pH range. These results indicate that the proposed sensor is convenient for practical applications of Zn2+ determination from actual samples.
3.4. Selectivity
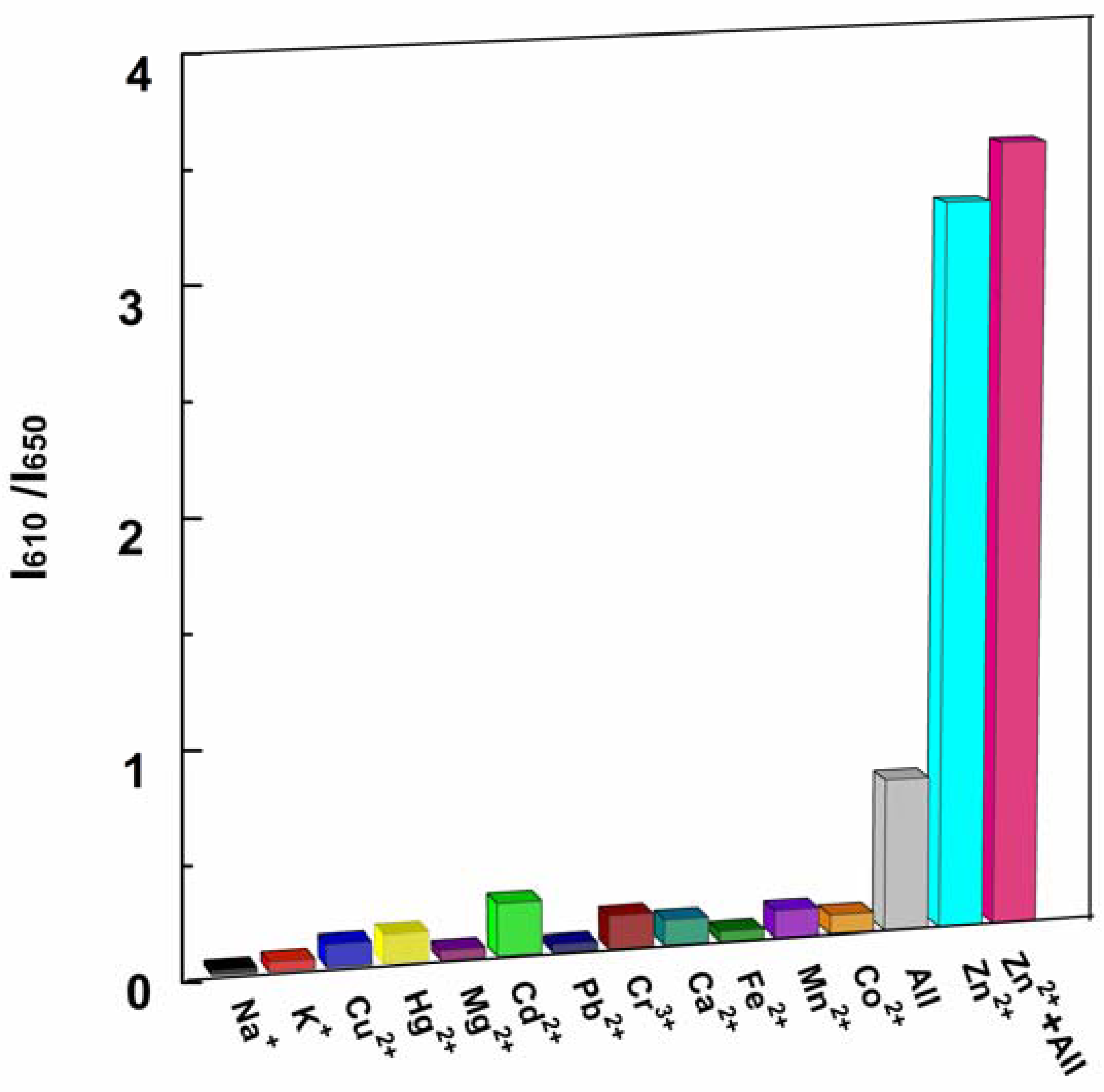
High selectivity is a necessary feature of excellent chemosensors. The selectivity of P1 to Zn2+ ions and competition with other metal ions were determined by fluorescence measurements. A series of metal ion interferences (1.0 × 103 μM) and their mixture were added to a solution of P1 (10 μM). Figure 6 indicated that only the addition of Zn2+ showed distinct ratiometric responses. Physiologically important metal ions found in living cells, such as Ca2+, Mg2+, Na+, Fe2+ and K+, were non-responsive to the probe. Most heavy and transition metal ions, such as Pb2+, Hg2+, and Cu2+, also showed no interference. More importantly, the addition of Cd2+, which is recognized as a typical competing ion of Zn2+ sensors [2,42,43], led to no obvious variation in the ratio of emission intensity. These results unambiguously demonstrate that the sensing of Zn2+ by P1 is hardly affected by common coexisting metal ions even when the concentration of these ions is 100 times higher than that of Zn2+ (see Figure 6 caption).
4. Conclusions
In conclusion, we have designed and synthesized a new ratiometric fluorescent chemosensor P1 that shows high selectivity, sensitivity to Zn2+, and the absence of interference from other competing cations, especially from Cd2+. P1 behaves like a tridentate ligand, in which Zn2+ is bound with three nitrogen atoms. Zn2+/P1 complexation quenches the fluorescence of porphyrin at 650 nm and induces a new fluorescence peak at 610 nm. Moreover, the chemosensor was pH independent under physiological conditions, indicating that it has valuable potential application in biological systems.
Acknowledgments
The authors gratefully acknowledge the financial support from the Education Department Foundation of Zhejiang Province of China (Grant No. Y201223312), the Foundation of Hangzhou Science and Technology Information Institute of China (Grant No. 20120433B26) and Scientific Research Foundation of Zhejiang University City College of China (Grant No. J-13001).
References
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar]
- Xie, G.Q.; Shi, Y.J.; Hou, F.P.; Liu, H.Y.; Huang, L.; Xi, P.X.; Chen, F.J.; Zeng, Z.Z. Highly selective fluorescent and colorimetric chemosensor for ZnII and its application in cell imaging. Eur. J. Inorg. Chem. 2012, 162, 327–332. [Google Scholar]
- Ajayaghosh, A.; Carol, P.; Sreejith, S. A ratiometric fluorescence probe for selective visual sensing of Zn2+. J. Am. Chem. Soc. 2005, 127, 14962–14963. [Google Scholar]
- Xu, Z.C.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar]
- Kimura, E.; Koike, T. Recent development of zinc-fluorophores. Chem. Soc. Rev. 1998, 27, 179–184. [Google Scholar]
- Zhou, X.Y.; Yu, B.R.; Guo, Y.L.; Tang, X.L.; Zhang, H.H.; Liu, W.S. Both visual and fluorescent sensor for Zn2+ based on quinoline platform. Inorg. Chem. 2010, 49, 4002–4007. [Google Scholar]
- Chen, X.Y.; Shi, J.; Li, Y.M.; Wang, F.L.; Wu, X.; Guo, Q.X.; Liu, L. Two-photon fluorescent probes of biological Zn(II) derived from 7-hydroxyquinoline. Org. Lett. 2009, 11, 4426–4429. [Google Scholar]
- Hirano, T.; Kikuchi, K.; Urano, Y.; Nagano, T. Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J. Am. Chem. Soc. 2002, 124, 6555–6562. [Google Scholar]
- Walkup, G.K.; Burdette, S.C.; Lippard, S.J.; Tsien, R.Y. A new cell-permeable fluorescent probe for Zn2+. J. Am. Chem. Soc. 2000, 122, 5644–5645. [Google Scholar]
- Xu, Z.C.; Liu, X.; Pan, J.; Spring, D.R. Coumarin-derived transformable fluorescent sensor for Zn2+. Chem. Commun. 2012, 48, 4764–4766. [Google Scholar]
- Li, L.; Dang, Y.Q.; Li, H.W.; Wang, B.; Wu, Y.Q. Fluorescent chemosensor based on Schiff base for selective detection of zinc(II) in aqueous solution. Tetrahedron Lett. 2010, 51, 618–621. [Google Scholar]
- Xu, Z.C.; Qian, X.H.; Cui, J.N.; Zhang, R. Exploiting the deprotonation mechanism for the design of ratiometric and colorimetric Zn2+ fluorescent chemosensor with a large red-shift in emission. Tetrahedron 2006, 62, 10117–10122. [Google Scholar]
- Shults, M.D.; Pearce, D.A.; Imperiali, B. Modular and tunable chemosensor scaffold for divalent zinc. J. Am. Chem. Soc. 2003, 125, 10591–10597. [Google Scholar]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin assemblies as chemical sensors. Coord. Chem. Rev. 1999, 190–192, 683–706. [Google Scholar]
- Gupta, V.K.; Chauhan, D.K.; Saini, V.K.; Agarwal, S.; Antonijevic, M.M.; Lang, H. A porphyrin based potentiometric sensor for Zn2+ determination. Sensors 2003, 3, 223–235. [Google Scholar]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A novel sensor for monitoring of iron(III) ions based on porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar]
- Zhang, Y.; Wang, H.; Yang, R.H. Colorimetric and fluorescent sensing of SCN− based on meso-tetraphenylporphyrin/meso-tetraphenylporphyrin cobalt(II) system. Sensors 2007, 7, 410–419. [Google Scholar]
- Ni, X.L.; Zeng, X.; Redshaw, C.; Yamato, T. Ratiometric fluorescent receptors for both Zn2+ and H2PO4− ions based on a pyrenyl-linked triazole-modified homooxacalix[3]arene: A potential molecular traffic signal with an R-S latch logic circuit. J. Org. Chem. 2011, 76, 5696–5702. [Google Scholar]
- Zhang, J.F.; Bhuniya, S.; Lee, Y.H.; Bae, C.; Lee, J.H.; Kim, J.S. Novel 2,2′-bipyridine-modified calix[4]arenes: ratiometric fluorescent chemosensors for Zn2+ ion. Tetrahedron Lett. 2010, 51, 3719–3723. [Google Scholar]
- Xu, Z.C.; Baek, K.H.; Kim, H.N.; Cui, J.N.; Qian, X.H.; Spring, D.R.; Shin, I.; Yoon, J. Zn2+ triggered amide tautomerization produces a highly Zn2+-selective, cell-permeable, and ratiometric fluorescent sensor. J. Am. Chem. Soc. 2010, 132, 601–610. [Google Scholar]
- Zhu, J.F.; Chan, W.H.; Lee, A.W.M. Both visual and ratiometric fluorescent sensor for Zn2+ based on spirobenzopyran platform. Tetrahedron Lett. 2012, 53, 2001–2004. [Google Scholar]
- Helal, A.; Kim, S.H.; Kim, H.S. Thiazole sulfonamide based ratiometric fluorescent chemosensor with a large spectral shift for zinc sensing. Tetrahedron 2010, 66, 9925–9932. [Google Scholar]
- Helal, A.; Kim, H.S. Thiazole-based chemosensor: synthesis and ratiometric fluorescence sensing of zinc. Tetrahedron Lett. 2009, 50, 5510–5515. [Google Scholar]
- Helal, A.; Lee, S.H.; Kim, S.H.; Kim, H.S. Dual-signaling fluorescent chemosensor based on bisthiazole derivatives. Tetrahedron Lett. 2010, 51, 3531–3535. [Google Scholar]
- Gao, C.J.; Jin, X.J.; Yan, X.H.; An, P.; Zhang, Y.; Liu, L.L.; Tian, H.; Liu, W.S.; Yao, X.J.; Tang, Y. A small molecular fluorescent sensor for highly selectivity of zinc ion. Sens. Actuators B Chem. 2013, 176, 775–781. [Google Scholar]
- Pizzotti, M.; Ugo, R.; Annoni, E.; Quici, S.; Ledoux-Rak, I.; Zerbi, G.; Zoppo, M.D.; Fantucci, P.; Invernizzi, I. A critical evaluation of EFISH and THG non-linear optical responses of asymmetrically substituted meso-tetraphenyl porphyrins and their metal complexes. Inorg. Chim. Acta. 2002, 340, 70–80. [Google Scholar]
- Xie, G.Q.; Xi, P.X.; Wang, X.; Zhao, X.F.; Huang, L.; Chen, F.J.; Wu, Y.J.; Yao, X.J.; Zeng, Z.Z. A highly zinc(II)-selective fluorescent sensor based on 8-aminoquinoline and its application in biological imaging. Eur. J. Inorg. Chem. 2011, 2011, 2927–2931. [Google Scholar]
- Liu, B.; Tian, H. A selective fluorescent ratiometric chemodosimeter for mercury ion. Chem. Commun. 2005, 3156–3158. [Google Scholar]
- Kim, J.B.; Leonard, J.J.; Longo, F.R. Mechanistic study of the synthesis and spectral properties of meso-tetraarylporphyrins. J. Am. Chem. Soc. 1972, 94, 3986–3992. [Google Scholar]
- Crosby, G.A.; Demas, J.N. Quantum efficiencies of transition-metal complexes. I. d-d luminescence. J. Am. Chem. Soc. 1970, 92, 7262–7270. [Google Scholar]
- Mashraqui, S.H.; Betkar, R.; Ghorpade, S.; Tripathi, S.; Britto, S. A new internal charge transfer probe for the highly selective detection of Zn(II) by means of dual colorimetric and fluorescent turn-on responses. Sens. Actuators B Chem. 2012, 174, 299–305. [Google Scholar]
- Wang, R.M.; Huang, S.B.; Zhao, N.; Chen, Z.N. A new Zn2+ chemosensor based on functionalized 8-hydroxylquinoline. Inorg. Chem. Commun. 2010, 13, 1432–1434. [Google Scholar]
- Ma, Q.J.; Zhang, X.B.; Zhao, X.H.; Gong, Y.J.; Tang, J.; Shen, G.L.; Yu, R.Q. A ratiometric fluorescent sensor for zinc ions based on covalently immobilized derivative of benzoxazole. Spectrochim. Acta Part A. 2009, 73, 687–693. [Google Scholar]
- Gong, Z.L.; Ge, F.; Zhao, B.X. Novel pyrazoline-based selective fluorescent sensor for Zn2+ in aqueous media. Sens. Actuators B Chem. 2011, 159, 148–153. [Google Scholar]
- Kong, L.; Chen, Y.; Ye, W.B.; Zhao, L.; Song, B.; Yang, J.X.; Tian, Y.P.; Tao, X.T. Synthesis, characterization, optical property of a bipyridine derivative and its application to the determine trace Zn2+ in water. Sens. Actuators B Chem. 2013, 177, 218–223. [Google Scholar]
- Helal, A.; Rashid, M.H.O.; Choi, C.H.; Kim, H.S. New regioisomeric naphthol-substituted thiazole based ratiometric fluorescence sensor for Zn2+ with a remarkable red shift in emission spectra. Tetrahedron 2012, 68, 647–653. [Google Scholar]
- Zhang, C.L.; Zhang, Y.M.; Chen, Y.C.; Xie, Z.J.; Liu, Z.P.; Dong, X.D.; He, W.J.; Shen, C.; Guo, Z.J. A sulfonamidoquinoline-derived Zn2+ fluorescent sensor with 1:1 Zn2+ binding stoichiometry. Inorg. Chem. Commun. 2011, 14, 304–307. [Google Scholar]
- Shen, K.; Yang, X.; Cheng, Y.X.; Zhu, C.J. A highly selective ratiometric fluorescent chemosensor for Zn2+ ion based on a polyimine macrocycle. Tetrahedron 2012, 68, 5719–5723. [Google Scholar]
- Huang, L.; Wang, X.; Xie, G.Q.; Xi, P.X.; Li, Z.P.; Xu, M.; Wu, Y.J.; Bai, D.C.; Zeng, Z.Z. A new rhodamine-based chemosensor for Cu2+ and the study of its behaviour in living cells. Dalton Trans. 2010, 39, 7894–7896. [Google Scholar]
- Kalimuthu, P.S.; John, A. Optochemical sensing of hydrogen chloride gas using meso-tetramesitylporphyrin deposited glass plate. Anal. Chim. Acta. 2008, 627, 247–253. [Google Scholar]
- Li, C.Y.; Zhang, X.B.; Dong, Y.Y.; Ma, Q.J.; Han, Z.X.; Zhao, Y.; Shen, G.L.; Yu, R.Q. A porphyrin derivative containing 2-(oxymethyl)pyridine units showing unexpected ratiometric fluorescent recognition of Zn2+ with high selectivity. Anal. Chim. Acta. 2008, 616, 214–221. [Google Scholar]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar]
Supplementary Files
A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity. Sensors 2013, 13, 3131-3141© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lv, Y.; Cao, M.; Li, J.; Wang, J. A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity. Sensors 2013, 13, 3131-3141. https://doi.org/10.3390/s130303131
Lv Y, Cao M, Li J, Wang J. A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity. Sensors. 2013; 13(3):3131-3141. https://doi.org/10.3390/s130303131
Chicago/Turabian StyleLv, Yuanyuan, Mingda Cao, Jiakai Li, and Junbo Wang. 2013. "A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity" Sensors 13, no. 3: 3131-3141. https://doi.org/10.3390/s130303131



