Optimization of Hydrogen Peroxide Detection for a Methyl Mercaptan Biosensor
Abstract
: Several kinds of modified carbon screen printed electrodes (CSPEs) for amperometric detection of hydrogen peroxide (H2O2) are presented in order to propose a methyl mercaptan (MM) biosensor. Unmodified, carbon nanotubes (CNTs), cobalt phthalocyanine (CoPC), Prussian blue (PB), and Os-wired HRP modified CSPE sensors were fabricated and tested to detect H2O2, applying a potential of +0.6 V, +0.6 V, +0.4 V, −0.2 V and −0.1 V (versus Ag/AgCl), respectively. The limits of detection of these electrodes for H2O2 were 3.1 μM, 1.3 μM, 71 nM, 1.3 μM, 13.7 nM, respectively. The results demonstrated that the Os-wired HRP modified CSPEs gives the lowest limit of detection (LOD) for H2O2 at a working potential as low as −0.1 V. Os-wired HRP is the optimum choice for establishment of a MM biosensor and gives a detection limit of 0.5 μM.1. Introduction
Methyl mercaptan (MM) is one of the volatile sulfur compounds (VSCs), which are known to be involved in halitosis (bad breath) [1,2] and periodontal diseases [2], and the predominant causative factor of noticeable oral malodor [3]. MM is also present in several other cases such as the bottle storage of wines [4]; wood-pulp mills, sewage treatment plants and factories producing jet fuel, pesticides and plastics [5]; and even in the atmosphere and on the ocean surface [6]. Consequently, MM detection is important in the dental, medical, food, environment and atmosphere fields.
A low-cost, sensitive and specific sensor for detecting MM could be an interesting alternative to conventional MM monitoring methods such as the use of a halimeter, an expensive device, in the dental field [7,8]. Biosensors to monitor MM have been described by Mitsubayashi et al. [9–12]. In their work, monoamine oxidase A (MAO-A) or flavin-containing monooxygenase (FMO) was used to catalytically oxidize MM, and the oxygen consumption induced by this reaction was monitored. Coupled with this system, a substrate regeneration cycle with ascorbic acid was carried out. However, a sensor for the detection of O2 depletion, which has the initially high current background of the oxygen electrode [13], is less sensitive than one for H2O2 measurement. To solve this problem and seek sensitive detection methods, our objective was to develop a MM biosensor coupled with sensitive hydrogen peroxide detection. Alcohol oxidase (AOX) is known to catalytically oxidize MM with production of formaldehyde, sulfide and H2O2 [14] according to the reaction:
Hydrogen peroxide generated during enzyme-catalyzed reactions can be electrochemically detected on modified/unmodified carbon matrixes [15–27]. In this work, unmodified carbon nanotube (CNT), cobalt phthalocyanine (CoPC), Prussian blue (PB), and Os-wired HRP modified screen printed electrode (CSPE) sensors were fabricated and tested to detect H2O2. Our aim was to seek the most sensitive and optimal detection method of H2O2 for a MM amperometric biosensor.
2. Experimental
2.1. Reagents
Hydrogen peroxide (H2O2, 30%, w/w), disodium hydrogen phosphate (Na2HPO4), potassium chloride (KCl), sodium chloride (NaCl), acetic acid (CH3COOH), sodium acetate trihydrate (CH3COONa·3H2O), potassium hexacyanoferrate (III) (K3Fe(CN)6), o-phenylenediamine (99.5%), bovine serum albumin (BSA, ≥96%) were obtained from Sigma-Aldrich (Lyon, France). The concentration of diluted H2O2 solutions was determined by the classic potassium permanganate titration method. Sulfuric acid (95%) (H2SO4) and NaH2PO4·2H2O were purchased from Prolabo (Briare, France). Hydrochloric acid (HCl) (37%) was obtained from Carlo Erba Reagenti (Milan, Italy). Peroxidase redox polymer (Os-wired HRP) was purchased from Bioanalytical Systems, Inc. (Gloucestershire, UK). Carbon pastes used for screen printed electrodes (Electrodag PE-410, 423SS and 6037SS) were obtained from Acheson (Plymouth, UK). A glycerolphtalic paint (Astral, France) was used as insulating layer. Transparent PVC sheets (200 mm × 100 mm × 0.5 mm) (SKK, Denzlingen, Germany) were used as screen-printing substrates. All chemicals were used without any further purification. All solutions were prepared using Milli-Q water.
2.2. Instrument
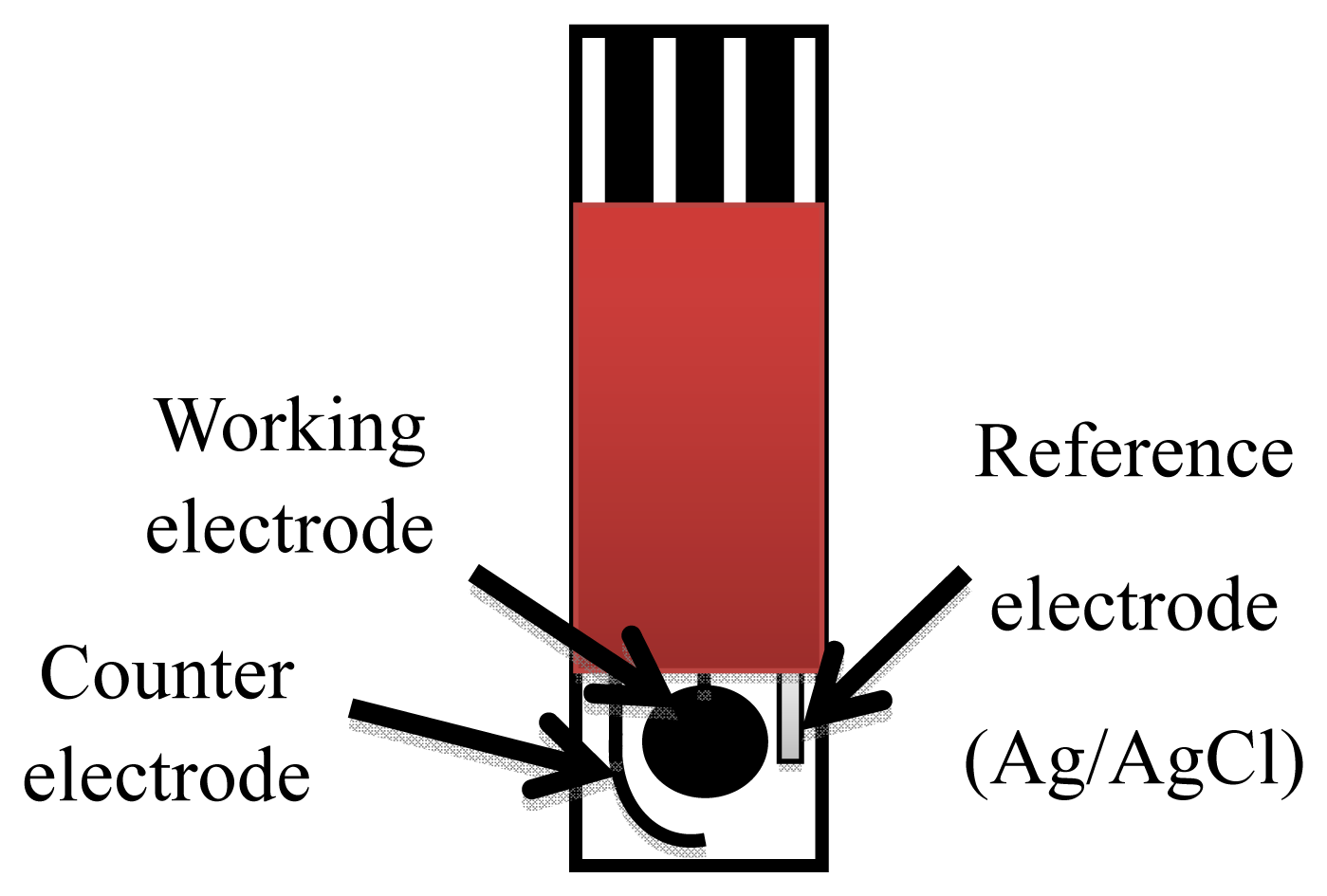
CSPEs were produced in the laboratory using a semi-automatic DEK 248 screen-printing system (DEK, Weymounth, UK). The working electrode was a 4 mm diameter disk, the auxiliary electrode was a 16 mm × 1.5 mm curved wire and the Ag/AgCl reference electrode was a 5 mm × 1.5 mm straight wire (Figure 1).
Cyclic voltammetry (CV) measurements, PB electrodeposition, PPD electropolymerisation and amperometric measurements were carried out on an AUTOLAB PGSTAT100 (Metrohm, Switzerland), using GPES v4.7 (Metrohm) as informatic interface. All potential values are reported versus Ag/AgCl. Amperometric measurements were performed in a 10 mL glass bath cell with magnetic stirring at room temperature.
2.3. Carbon Screen Printed Electrode Modifications
2.3.1. Preparation of CNT Modified CSPEs
CNT modified CSPEs were prepared as described in the work of Silveira et al. [28]. Briefly, 10 μL of 0.3 mg/mL SWCNT water dispersion were successively deposited on the CSPEs working electrode, drying each layer one by one under vacuum. The electrodes were then washed with water.
2.3.2. Preparation of CoPC Modified CSPEs
Cobalt-phtalocyanine-modified paste was purchased from Gwent Electronic Materials, Ltd. (Gwent, UK) and modifications were performed on working electrode by the DEK screen-printing system.
2.3.3. Preparation of PB/PPD Modified CSPEs
The PB film was first deposited by covering the CSPEs with a solution containing 2.5 mM FeCl3, 2.5 mM K3Fe(CN)6, 0.1 M KCl and 0.1 M HCl and applying a potential +0.4 V versus Ag/AgCl for 40 s. Then the PB film was activated by covering the electrode by a solution containing 0.1 M KCl and 0.1 M HCl, electrochemically cycling for 20 cycles between −0.05 V and 0.35 V versus Ag/AgCl at a scan rate of 50 mV·s−1. After washing with distilled water, it was dried for 1 h at 100 °C in oven. To improve the stability and selectivity properties of the PB electrodes, the electropolymerisation of a poly- (o-phenylenediamine) (PPD) coat was formed. The PPD layer was deposited by electrochemically cycling the PB modified electrode with potential between −0.5 V and 0.7 V versus Ag/AgCl at a scan rate of 50 mV·s−1 in deaerated 0.1 M, pH 5.0 acetate buffer solution containing 0.5 mM o-phenylenediamine under a stream of nitrogen [29].
2.3.4. Preparation of Os Wired HRP (Os-HRP) Modified CSPEs
For the Os-HRP modified CSPEs, 10 μL 0.1 M phosphate buffer solution, pH 7.5, containing 10% (v/v) Os-HRP was deposited on the surface of CSPEs. It was allowed to dry at room temperature for 2 h. It was thoroughly washed with buffer before use.
3. Results and Discussion
3.1. H2O2 Detection with Unmodified CSPEs
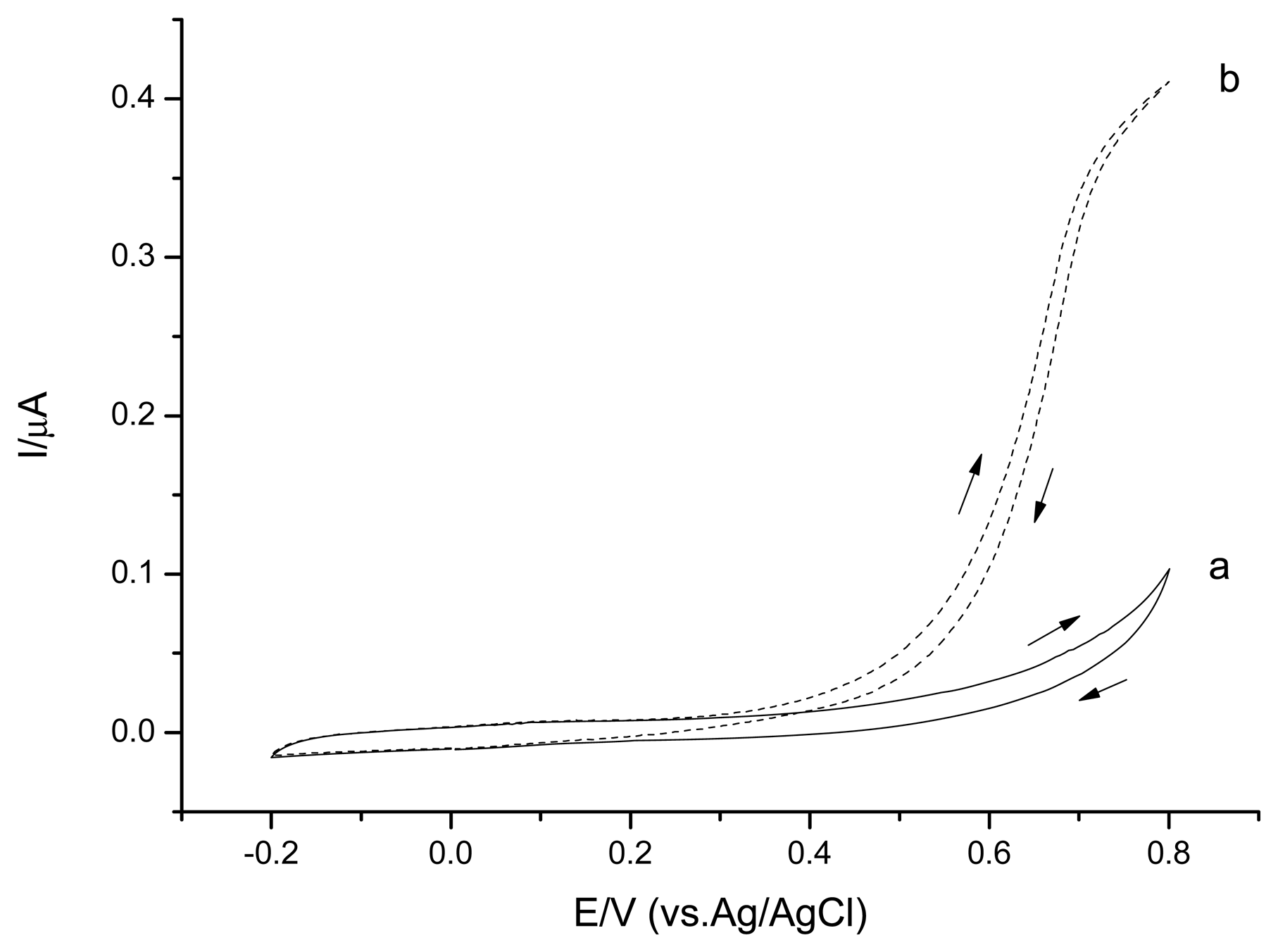
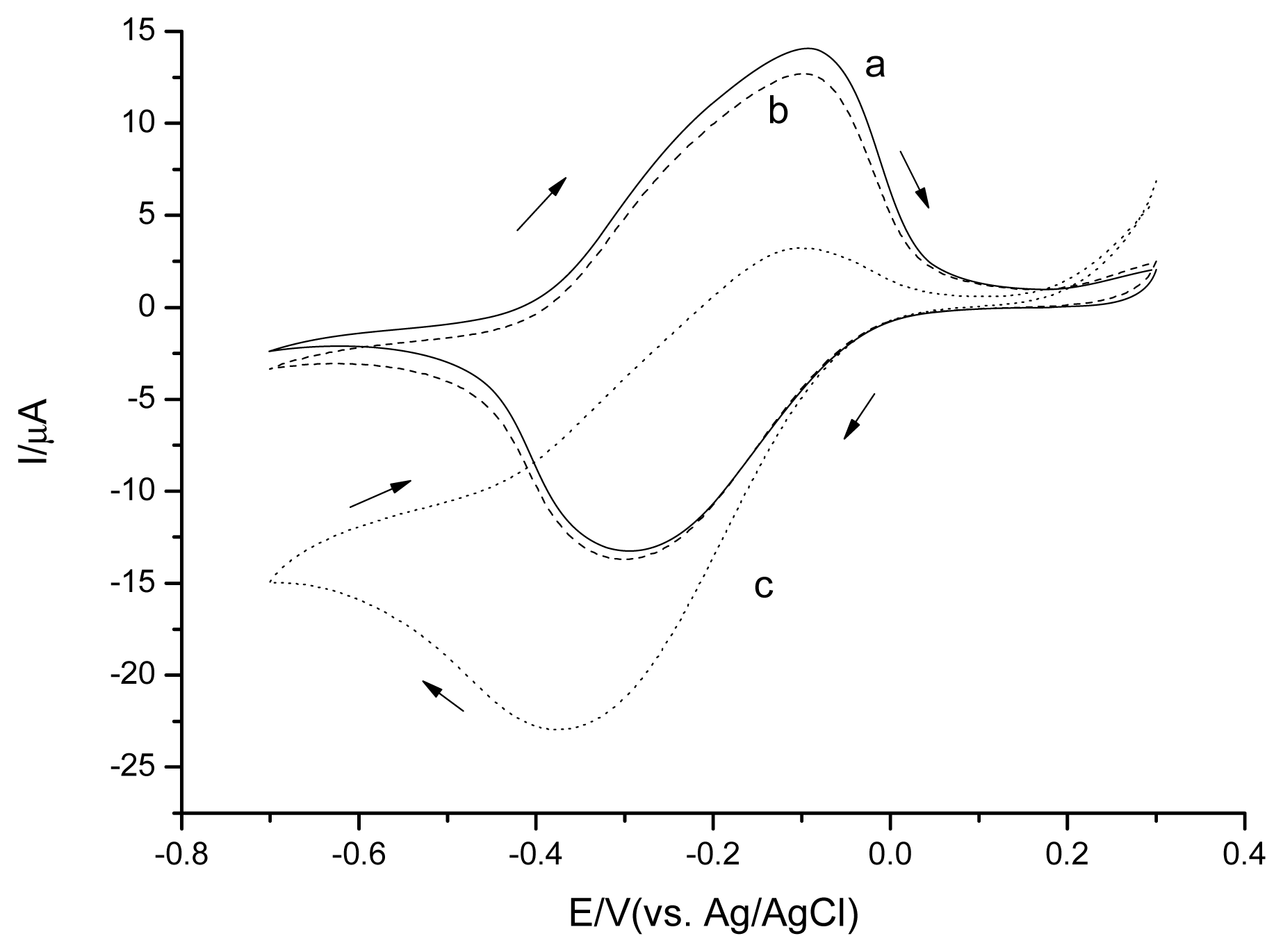
The cyclic voltammetry studies were performed in 0.1 M phosphate buffer solution, pH 7.5 to investigate the CSPEs' electrochemical behavior (Figure 2). CSPEs showed no obvious peak in the absence of H2O2 in the potential range from −0.2 V∼0.8 V versus Ag/AgCl. In the presence of H2O2, CSPEs started to perform current response at potential around +0.3 V versus Ag/AgCl, indicating the onset potential of the H2O2 electrooxidation.
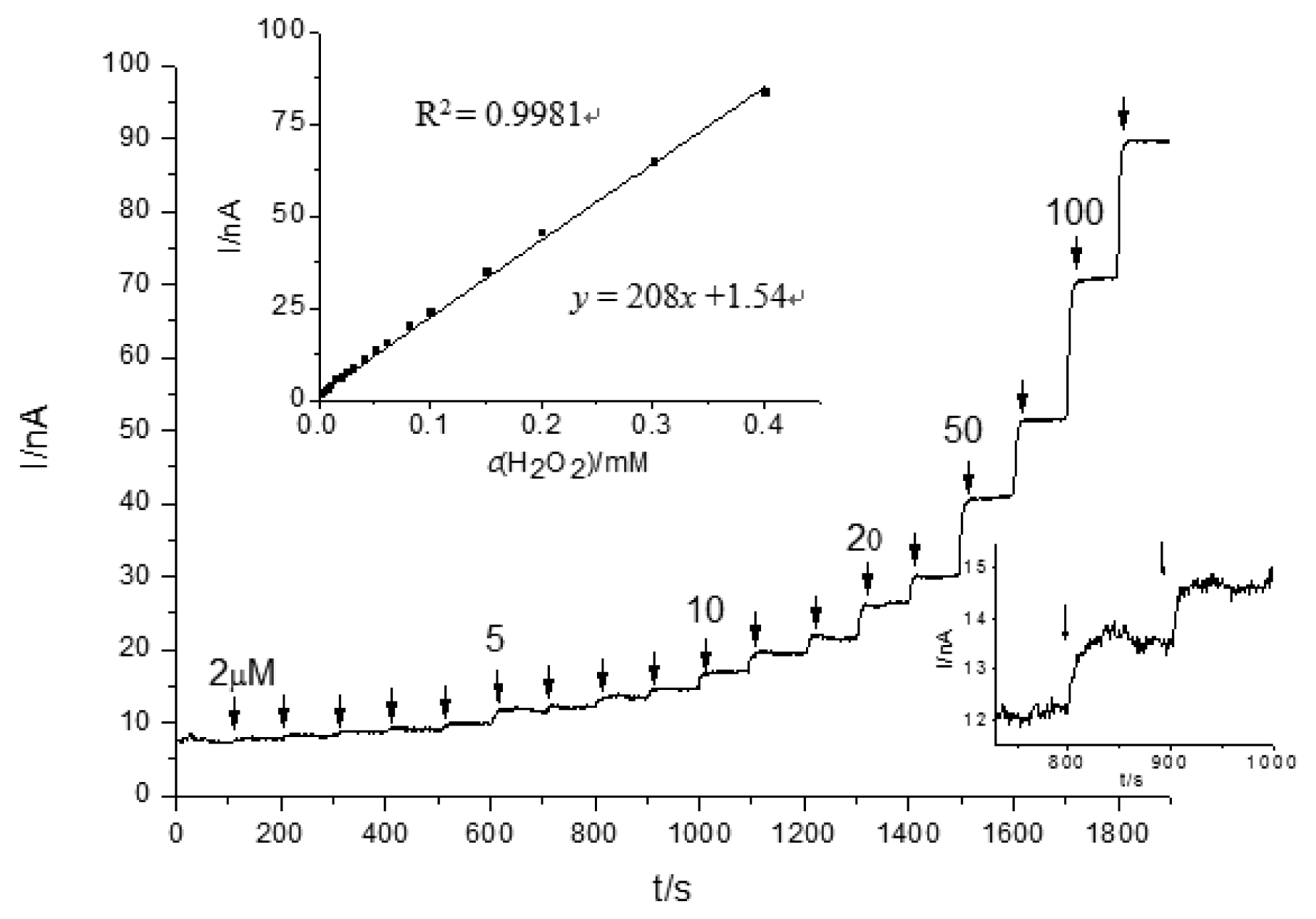
For the investigation of H2O2 limit detection, chronoamperometry experiments were carried out with several concentrations of H2O2 injected into the stirred bath cell (Figure 3). For unmodified CSPEs, the H2O2 detection limit was 3.1 μM (S/N = 3) applying a +0.6 V potential versus Ag/AgCl, and the current response slope of the calibration curve was 0.208 μA/mM. To investigate the reproducibility, three parallel measurements with 0.1 mM H2O2 revealed a relative standard deviation (RSD) of 12.1%. The high RSD observed of unmodified CPSEs is likely related to the marked differences in the real active electrode area, which is difficult to handle and adjust.
3.2. H2O2 Detection with CNT/CSPEs
Both CSPEs (Figure 2) and CNT/CSPEs (Figure 4) showed a H2O2 oxidation peak in the cyclic voltammetry experiments for the studied potential range. The onset potential of the H2O2 electrooxidation for CNT/CSPEs was around +0.2 V versus Ag/AgCl, the detection limit was 1.3 μM (S/N = 3) applying a positive potential of +0.6 V versus Ag/AgCl, and the current response slope of CNT/CSPEs for H2O2 was 32.1 μA/mM. To investigate the reproducibility, three parallel measurements with 0.1 mM H2O2 revealed a RSD of 19.7%.
Both unmodified CSPEs and CNT are intrinsically carbon. Compared to unmodified CSPEs, detections of H2O2 for CNT/CSPEs need lower oxidation potential (CSPEs, +0.3 V; CNT/CSPEs, +0.2 V), and have lower detection limit (CSPEs, 3.1 μM; CNT/CSPEs, 1.3 μM) with higher current response (CSPEs, 0.208 μA/mM; CNT/CSPEs, 32.1 μA/mM). The increased current response may arise from the large electric active area and a thin, porous diffusion layer [30]; the reasons of lower onset oxidation potential and lower detection limit are still controversial [31], because CNT may contain metal impurities derived from the catalysts used for their growth [32,33]. In a sense, CNT/CSPEs could be more favorable than unmodified CSPEs for H2O2 detection.
3.3. H2O2 Detection with CoPC/CSPEs
To investigate the electrochemical behavior of CoPC/CSPEs in phosphate buffer solution, cyclic voltammetry experiments were carried out in the potential range of −0.2 V∼0.8 V versus Ag/AgCl (Figure 5). The presence of a well-defined oxidation current peak at around +0.3 V versus Ag/AgCl is consistent with the following reaction [21]:
The reaction can be described by a chemical-electrochemical (CE) mechanism [21]: H2O2 chemically reduces Co2+ to Co+ and its subsequent electrochemical re-oxidation is observed as an oxidation peak. Consequently, this peak is used for the quantification of H2O2. For CoPC/CSPEs, the H2O2 detection limit was calculated as 71 nM (S/N = 3) applying a positive potential of +0.4 V versus Ag/AgCl in chronoamperometry experiments, and the slope of the calibration curve was 3.7 μA/mM by. Three parallel measurements with 10 μM H2O2 reveal a RSD of 2.4%, indicating a good reproducibility for this sensor.
3.4. H2O2 Detection with PPD/PB/CSPEs
PB is known to have a high solubility in neutral and basic solutions [34,35], consequently the PB-modified electrodes may have stability problems under our conditions. To improve the stability and selectivity of the PB electrodes a PPD coat is formed. In order to assess the effect of the PPD coat on the stability of the PB modified electrodes, cyclic voltammetry experiments were carried out in 0.1 M phosphate buffer pH 7.5 (not shown in this paper).
Without PPD layer coating, PB dissolved in solution, resulting in a CV current decrease in the scanning process. After 20 scan cycles, the PB/CSPEs CV's performance were similar to the unmodified CSPEs (Figure 2). With PPD layer coating, the response of redox peaks of PB reduced slightly even after 50 scan cycles, indicating that the PPD layer stabilized the PB. To investigate the electrochemical performance of PPD/PB/CSPEs H2O2 was added to the solution (Figure 6).
In the phosphate solution, the redox peaks correspond to the reduction of Prussian blue and oxidation of Prussian white as [36]:
In the presence of H2O2, the electrocatalytical reductive reaction of PB towards to H2O2 can be described as:
For PPD/PB/CSPEs, the H2O2 detection limit was 1.3 μM (S/N = 3) for a cathode potential of −0.2 V and the current response slope was −33 μA/mM. Three parallel measurements with 10 μM H2O2 revealed a RSD = 4.1%. The lower RSDs observed of CoPC/CSPEs and PPD/PB/CSPEs than for unmodified CPSEs and CNT/CSPEs are likely related to the better control of mediator deposition.
3.5. H2O2 Detection with Os-HRP/CSPEs
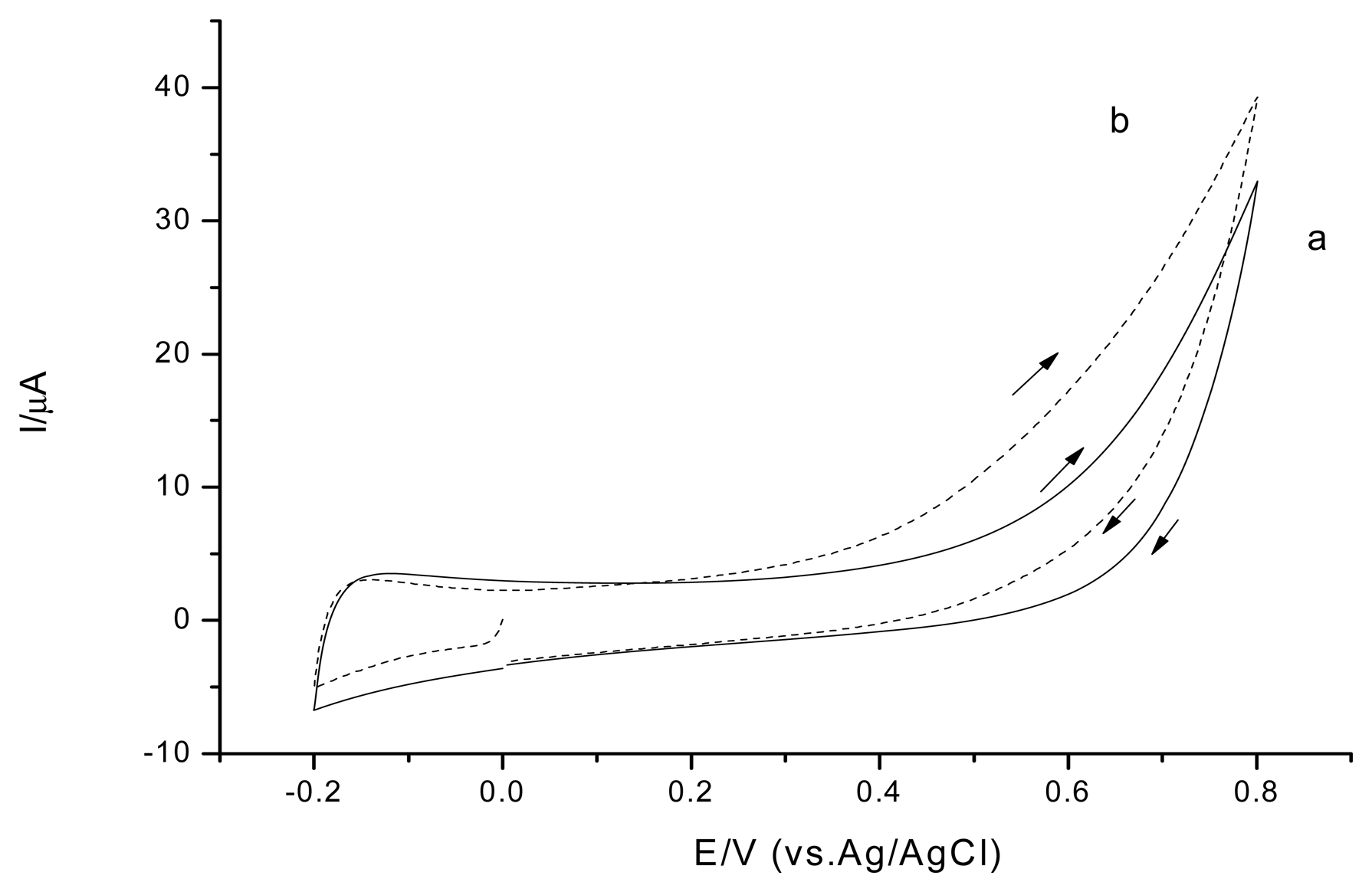
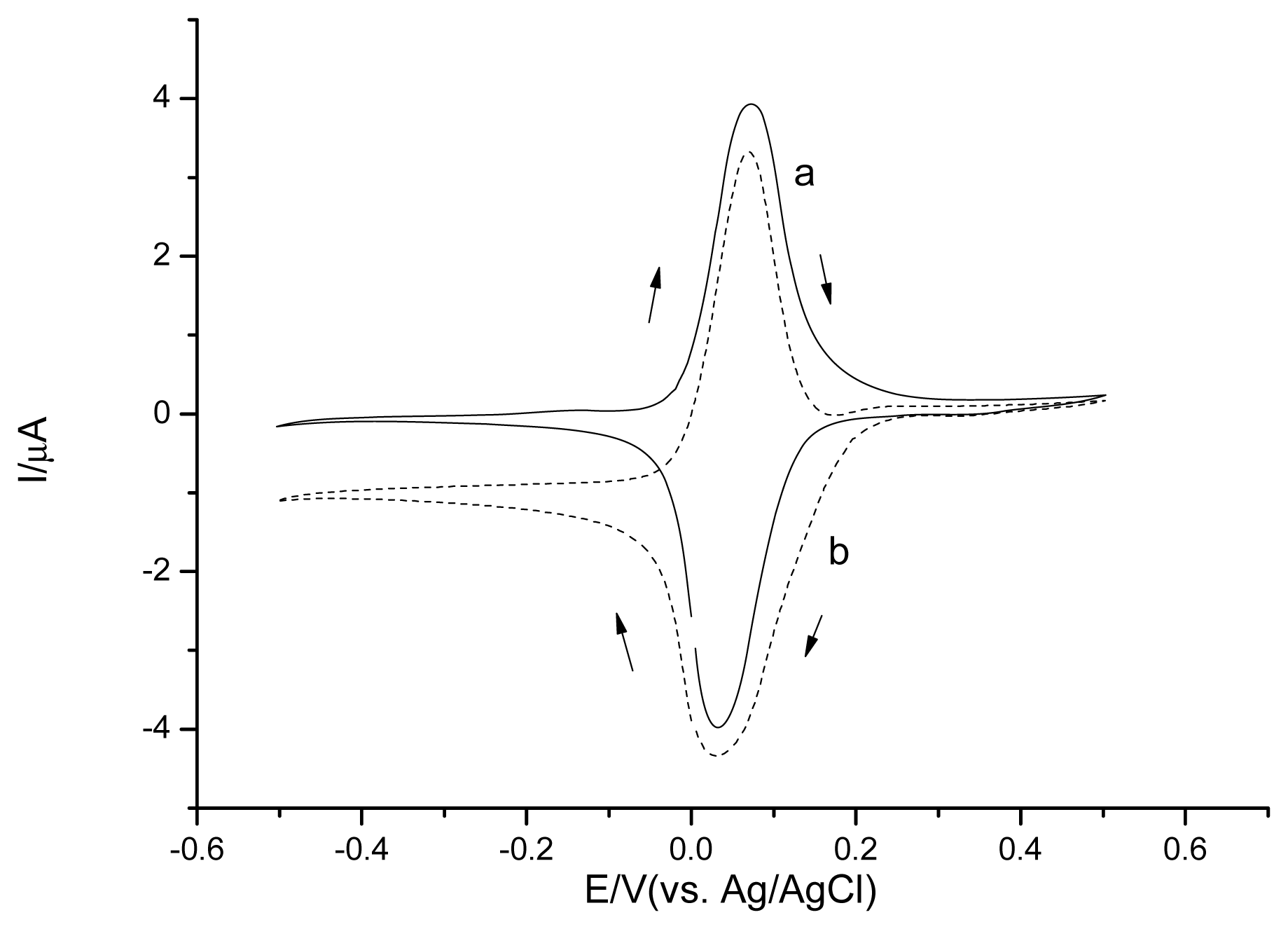
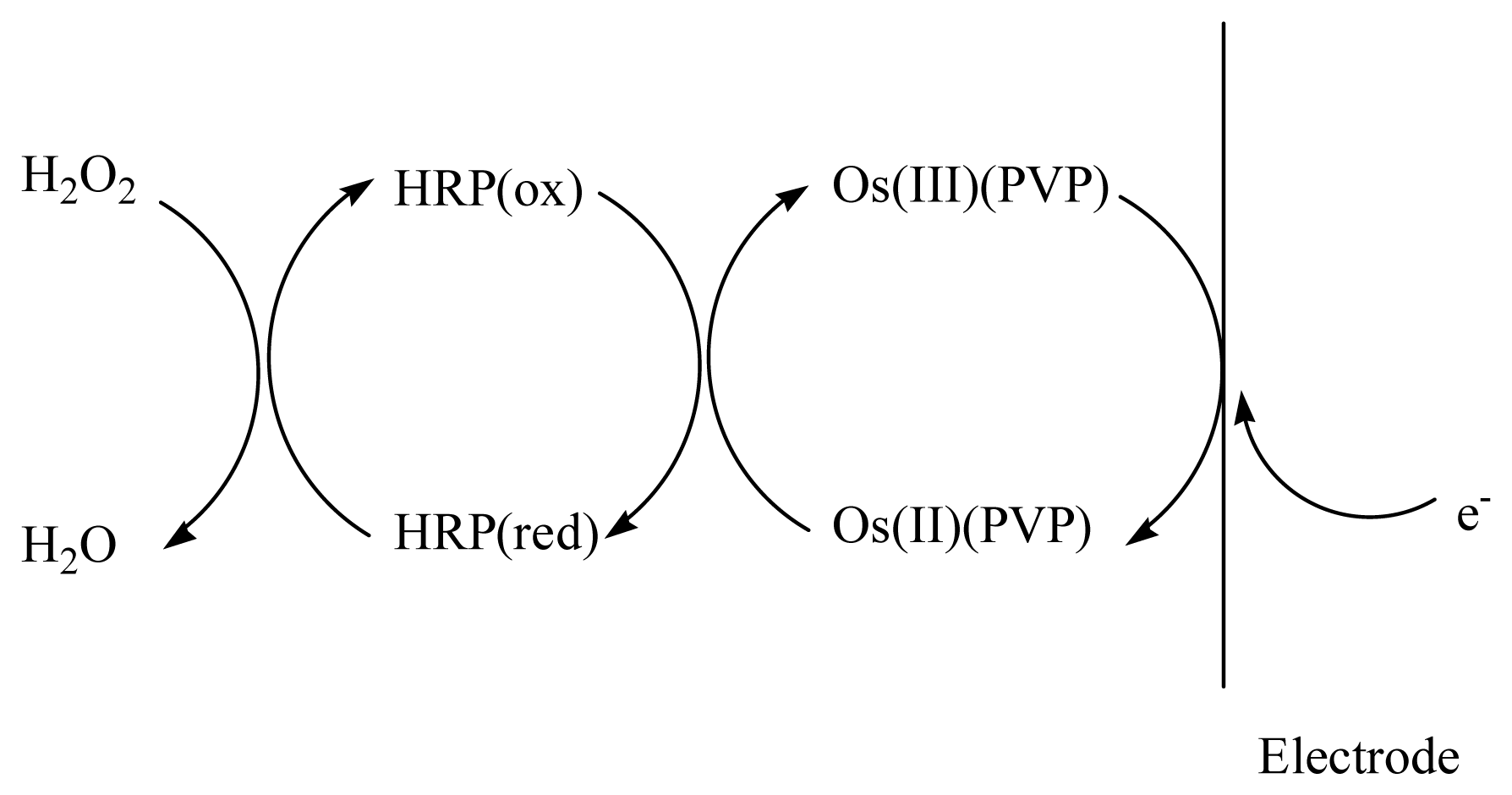
The Os-HRP/CSPEs show a couple of stable and well-defined redox peaks at around +30 mV and +70 mV at a scan rate of 20 mV·s−1 (Figure 7 curve a). In the presence of H2O2 (Figure 7 curve b), the electrocatalytical reductive reaction process of Os-HRP towards to H2O2 can be described by Scheme 1.
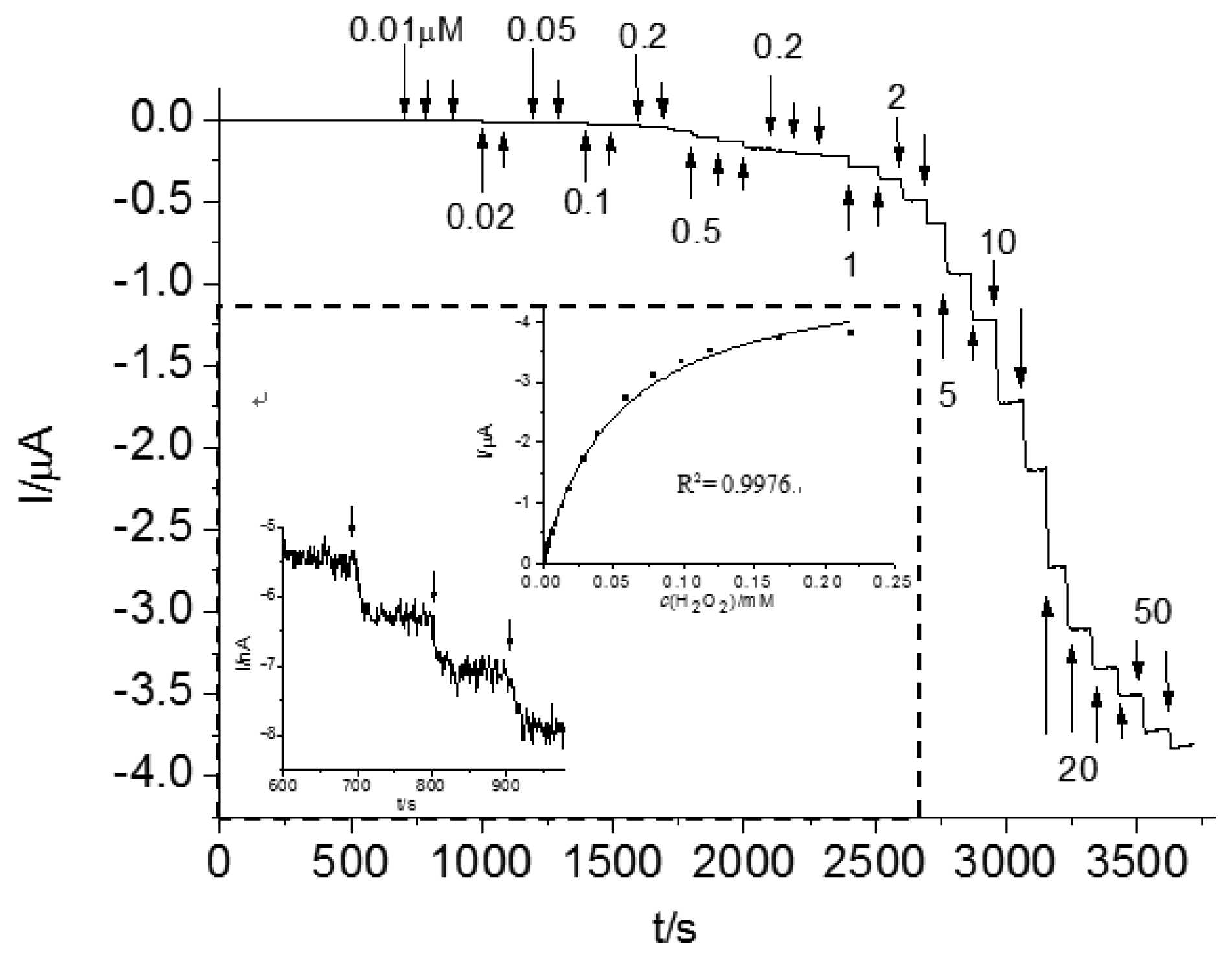
For Os-HRP/CSPEs, the H2O2 detection limit was 13.7 nM (S/N = 3) at a cathode potential −0.1 V versus Ag/AgCl (Figure 8). According to the Michaelis-Menten equation, the calculated apparent KM,p, from the curve fitting is 53.5 μM. This low KM,p value indicates Os-HRP's high affinity and high effective conversion for the H2O2 substrate and a favorable electron-transfer rate with the osmium mediator. A linear range was obtained until 25 μM. Three parallel measurements with 1 μM H2O2 revealed a RSD of 1.3%, which indicates a good reproducibility. This may be ascribed to the application of a low cathode potential to avoid the interferences with electro-active species. In addition, Os-HRP/CSPEs can be the most specific in detection of H2O2 because of the specificity of the reaction between HRP and H2O2.
The comparison of the amperometric analytical behavior to H2O2 of the five kinds of electrodes developed in this study is summarized in Table 1. It shows that the Os-HRP/CSPEs are the most sensitive electrodes with a low reduction potential applied for H2O2 detection. This is due to the specific, sensitive and rapid turnover of Os-HRP to H2O2.
Interference of electro-active species [25,37–39] is often encountered when using amperometric biosensors and applying a high potential in real samples. The decrease of the applied potential can be effective to avoid a lot of electrochemical interferences. With this consideration, PPD/PB/CSPEs and Os-HRP/CSPEs are used to combine with alcohol oxidase (AOX) in bovine serum albumin matrix to detect methyl mercaptan (MM) applying a low potential in the aqueous phase. The limit of detection of AOX/PPD/PB/CSPEs to MM is 10 μM; of AOX/Os-HRP/CSPEs to MM is 0.5 μM. For AOX/Os-HRP/CSPEs, the calibration curve of the response to MM is linear in the concentration range 0∼15 μM with a good correlation with the classical analytical method. We are working on the stability of the biosensor which is the crucial point to improve its accuracy and reliability. Consequently, Os-HRP/CSPEs are combined with alcohol oxidase an optimum method which gives the best sensitivity in methyl mercaptan detection.
4. Conclusions
Five kinds of modified carbon screen printed electrodes applied for H2O2 amperometric detection for MM biosensors were presented in this work. In comparison, and despite a worse reproducibility, CNT/CSPEs are a better choice than unmodified CPSEs in H2O2 detection resulting from their lowest detection limit, lowest onset oxidation potential and highest current response of CNT/CSPEs. However, the applied potential of +0.6 V versus Ag/AgCl is too positive to avoid the interference of electro-active species. CoPC/CSPEs and PPD/PB/CSPEs are also a good choice in H2O2 detection because of their low applied potential, low detection limit and good reproducibility. Os-HRP/CSPEs display the lowest detection limit and the best operational reproducibility towards H2O2. With the cathode potential applied and the use of HRP, Os-HRP/CSPEs can avoid the interference of electro-active species and be specific for H2O2 detection. The Os-wired HRP modified screen printed electrode is the optimum method we used to combine with alcohol oxidase in a methyl mercaptan biosensor, usable in both aqueous and gaseous phase detection.
Acknowledgments
Zhan-Hong Li thanks China Scholarship Council (CSC) for his financial support.
References
- Tonzetich, J.; Carpenter, P.A. Production of volatile sulphur compounds from cysteine, cystine and methionine by human dental plague. Arch. Oral Biol. 1971, 16, 599–607. [Google Scholar]
- Tonzetich, J. Production and origin of oral malodor: A review of mechanisms and methods of analysis. J. Periodontol. 1977, 48, 13–20. [Google Scholar]
- Awano, S.; Koshimune, S.; Kurihara, E.; Gohara, K.; Sakai, A.; Soh, I.; Hamasaki, T.; Ansai, T.; Takehara, T. The assessment of methyl mercaptan, an important clinical marker for the diagnosis of oral malodor. J. Dent. 2004, 32, 555–559. [Google Scholar]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.P.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-Mercaptohexanol, Hydrogen Sulfide, and Methyl Mercaptan during bottle storage of sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar]
- ATSDR. Toxicological Profile for Methyl Mercaptan; Agency for Toxic Substances and Disease Registry (ATSDR) US Public Health Service: Atlanta, GA, USA, 1992. [Google Scholar]
- Bates, T.S.; Lamb, B.K.; Guenther, A.; Dignon, J.; Stoiber, R.E. Sulfur emissions to the atmosphere from natural sources. J. Atmos. Chem. 1992, 14, 315–337. [Google Scholar]
- Rosenberg, M.; Kulkarni, G.V.; Bosy, A.; McCulloch, C.A. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J. Dent. Res. 1991, 70, 1436–1440. [Google Scholar]
- Rosenberg, M.; Septon, I.; Eli, I.; Bar-Ness, R.; Gelernter, I.; Brenner, S.; Gabbay, J. Halitosis measurement by an industrial sulphide monitor. J. Periodontol. 1991, 62, 487–489. [Google Scholar]
- Mitsubayashi, K.; Hashimoto, Y. Bioelectronic nose for methyl mercaptan vapor using xenobiotic metabolizing enzyme: Flavin-containing monooxygenase. Sens. Actuators B Chem. 2002, 83, 35–40. [Google Scholar]
- Minamide, T.; Mitsubayashi, K.; Jaffrezic-Renault, N.; Hibi, K.; Endo, H.; Saito, H. Bioelectronic detector with monoamine oxidase for halitosis monitoring. Analyst 2005, 130, 1490–1494. [Google Scholar]
- Minamide, T.; Mitsubayashi, K.; Saito, H. Bioelectronic sniffer with monoamine oxidase for methyl mercaptan vapor. Sens. Actuators B Chem. 2005, 108, 639–645. [Google Scholar]
- Mitsubayashi, K.; Minamide, T.; Otsuka, K.; Kudo, H.; Saito, H. Optical bio-sniffer for methyl mercaptan in halitosis. Anal. Chim. Acta 2006, 573, 75–80. [Google Scholar]
- Blum, L.J.; Coulet, P.R. Biosensor Principles and Applications; Marcel Dekker, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Suylen, G.M.H.; Large, P.J.; Dijken, J.P.V.; Kuenen, J.G. Methyl mercaptan oxidase, a key enzyme in the metabolism of methylated sulphur compounds by hyphomicvobium EG. J. Gen. Microbiol. 1987, 133, 2989–2997. [Google Scholar]
- Wang, J.; Musameh, M.; Lin, Y. Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review, trac-trend. Anal. Chem. 2006, 25, 480–489. [Google Scholar]
- Wang, J.; Musameh, M. Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal. Chem. 2003, 75, 2075–2079. [Google Scholar]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanal 2005, 17, 7–14. [Google Scholar]
- Wang, J.; Golden, T.; Li, R. Cobalt phthalocyanine/cellulose acetate chemically modified electrodes for electrochemical detection in flowing streams. Multifunctional operation based upon the coupling of electrocatalysis and permselectivity. Anal. Chem. 1988, 60, 1642–1645. [Google Scholar]
- Salimi, A.; Hallaj, R.; Soltanian, S.; Mamkhezri, H. Nanomolar detection of hydrogen peroxide on glassy carbon electrode modified with electrodeposited cobalt oxide nanoparticles. Anal. Chim. Acta 2007, 594, 24–31. [Google Scholar]
- Gilmartin, M.A.T.; Hart, J.P.; Birch, B.J. Development of amperometric sensors for uric acid based on chemically modified graphite-epoxy resin and screen-printed electrodes containing cobalt phthalocyanine. Analyst 1994, 119, 243–252. [Google Scholar]
- Gilmartin, M.A.T.; Ewen, R.J.; Hart, J.P.; Honeybourne, C.L. Voltammetric and photoelectron spectral elucidation of the electrocatalytic oxidation of hydrogen peroxide at screen-printed carbon electrodes chemically modified with cobalt phthalocyanine. Electroanal 1995, 7, 547–555. [Google Scholar]
- Ricci, F. Prussian Blue and enzyme bulk-modified screen-printed electrodes for hydrogen peroxide and glucose determination with improved storage and operational stability. Anal. Chim. Acta 2003, 485, 111–120. [Google Scholar]
- Ricci, F.; Amine, A.; Palleschi, G.; Moscone, D. Prussian Blue based screen printed biosensors with improved characteristics of long-term lifetime and pH stability. Biosens. Bioelectron. 2003, 18, 165–174. [Google Scholar]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar]
- Garguilo, M.G.; Nhan, H.; Proctor, A.; Michael, A.C. Amperometric sensors for peroxide, choline, and acetylcholine based on electron transfer between horseradish peroxidase and a redox polymer. Anal. Chem. 1993, 65, 523–528. [Google Scholar]
- Vreeke, M.; Maidan, R.; Heller, A. Hydrogen peroxide and .beta.-nicotinamide adenine dinucleotide sensing amperometric electrodes based on electrical connection of horseradish peroxidase redox centers to electrodes through a three-dimensional electron relaying polymer network. Anal. Chem. 1992, 64, 3084–3090. [Google Scholar]
- Silveira, C.M.; Baur, J.; Holzinger, M.; Moura, J.J.G.; Cosnier, S.; Almeida, M.G. Enhanced direct electron transfer of a multihemic nitrite reductase on single-walled carbon nanotube modified electrodes. Electroanal 2010, 22, 2973–2978. [Google Scholar]
- Lobo, M.J.; Miranda, A.J.; Tipez-Fonseca, J.M.; Tuih, P. Electrocatalytic detection of nicotinamide coenzymes by poly(o-aminophenol)- and poly(o-phenylenediamine)-modified carbon paste electrodes. Anal. Chim. Acta 1996, 325, 33–42. [Google Scholar]
- Scott, C.L.; Pumera, M. Carbon nanotubes can exhibit negative effects in electroanalysis due to presence of nanographite impurities. Electrochem. Commun. 2011, 13, 426–428. [Google Scholar]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Edit. 2010, 49, 2114–2138. [Google Scholar]
- Šljukić, B.; Banks, C.E.; Compton, R.G. Iron oxide particles are the active sites for hydrogen peroxide sensing at multiwalled carbon nanotube modified electrodes. Nano Lett. 2006, 6, 1556–1558. [Google Scholar]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Edit. 2006, 45, 2533–2537. [Google Scholar]
- Feldman, B.J.; Murray, R.W. Electron diffusion in wet and dry Prussian blue films on interdigitated array electrodes. Inorg. Chem. 1987, 26, 1702–1708. [Google Scholar]
- Stilwell, D.E.; Park, K.H.; Miles, M.H. Electrochemical studies of the factors influencing the cycle stability of Prussian Blue films. J. Appl. Electrochem. 1992, 22, 325–331. [Google Scholar]
- Haghighi, B.; Hamidi, H.; Gorton, L. Electrochemical behavior and application of Prussian Blue nanoparticle modified graphite electrode. Sens. Actuators B Chem. 2010, 147, 270–276. [Google Scholar]
- Palmisano, F.; Zambonin, P.G. Ascorbic acid interferences in hydrogen peroxide detecting biosensors based on electrochemically immobilized enzymes. Anal. Chem. 1993, 65, 2690–2692. [Google Scholar]
- Yamamoto, K.; Ohgaru, T.; Torimura, M.; Kinoshita, H.; Kano, K.; Ikeda, T. Highly-sensitive flow injection determination of hydrogen peroxide with a peroxidase-immobilized electrode and its application to clinical chemistry. Anal. Chim. Acta 2000, 406, 201–207. [Google Scholar]
- Palleschi, G.; Ali Nabi Rahni, M.; Lubrano, G.J.; Ngwainbi, J.N.; Guilbault, G.G. A study of interferences in glucose measurements in blood by hydrogen peroxide based glucose probes. Anal. Biochem. 1986, 159, 114–121. [Google Scholar]

| Type of Electrodes | Potential Applied (V) | LOD (S/N = 3) | RSD (Tested Concentration of H2O2) |
|---|---|---|---|
| CSPEs | +0.6 | 3.1 μM | 12.1% (0.1 mM) |
| CNT/CSPEs | +0.6 | 1.3 μM | 19.7% (0.1 mM) |
| CoPC/CSPEs | +0.4 | 71 nM | 2.4% (10 μM) |
| PPD/PB/CSPEs | −0.2 | 1.3 μM | 4.1% (10 μM) |
| Os-HRP/CSPEs | −0.1 | 13.7 nM | 1.3% (1 μM) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Z.-H.; Guedri, H.; Viguier, B.; Sun, S.-G.; Marty, J.-L. Optimization of Hydrogen Peroxide Detection for a Methyl Mercaptan Biosensor. Sensors 2013, 13, 5028-5039. https://doi.org/10.3390/s130405028
Li Z-H, Guedri H, Viguier B, Sun S-G, Marty J-L. Optimization of Hydrogen Peroxide Detection for a Methyl Mercaptan Biosensor. Sensors. 2013; 13(4):5028-5039. https://doi.org/10.3390/s130405028
Chicago/Turabian StyleLi, Zhan-Hong, Houssemeddine Guedri, Bruno Viguier, Shi-Gang Sun, and Jean-Louis Marty. 2013. "Optimization of Hydrogen Peroxide Detection for a Methyl Mercaptan Biosensor" Sensors 13, no. 4: 5028-5039. https://doi.org/10.3390/s130405028





