A Molecularly Imprinted Polymer with Incorporated Graphene Oxide for Electrochemical Determination of Quercetin
Abstract
: The molecularly imprinted polymer based on polypyrrole film with incorporated graphene oxide was fabricated and used for electrochemical determination of quercetin. The electrochemical behavior of quercetin on the modified electrode was studied in detail using differential pulse voltammetry. The oxidation peak current of quercetin in B-R buffer solution (pH = 3.5) at the modified electrode was regressed with the concentration in the range from 6.0 × 10−7 to 1.5 × 10−5 mol/L (r2 = 0.997) with a detection limit of 4.8 × 10−8 mol/L (S/N = 3). This electrode showed good stability and reproducibility. In the above mentioned range, rutin or morin which has similar structures and at the same concentration as quercetin did not interfere with the determination of quercetin. The applicability of the method for complex matrix analysis was also evaluated.1. Introduction
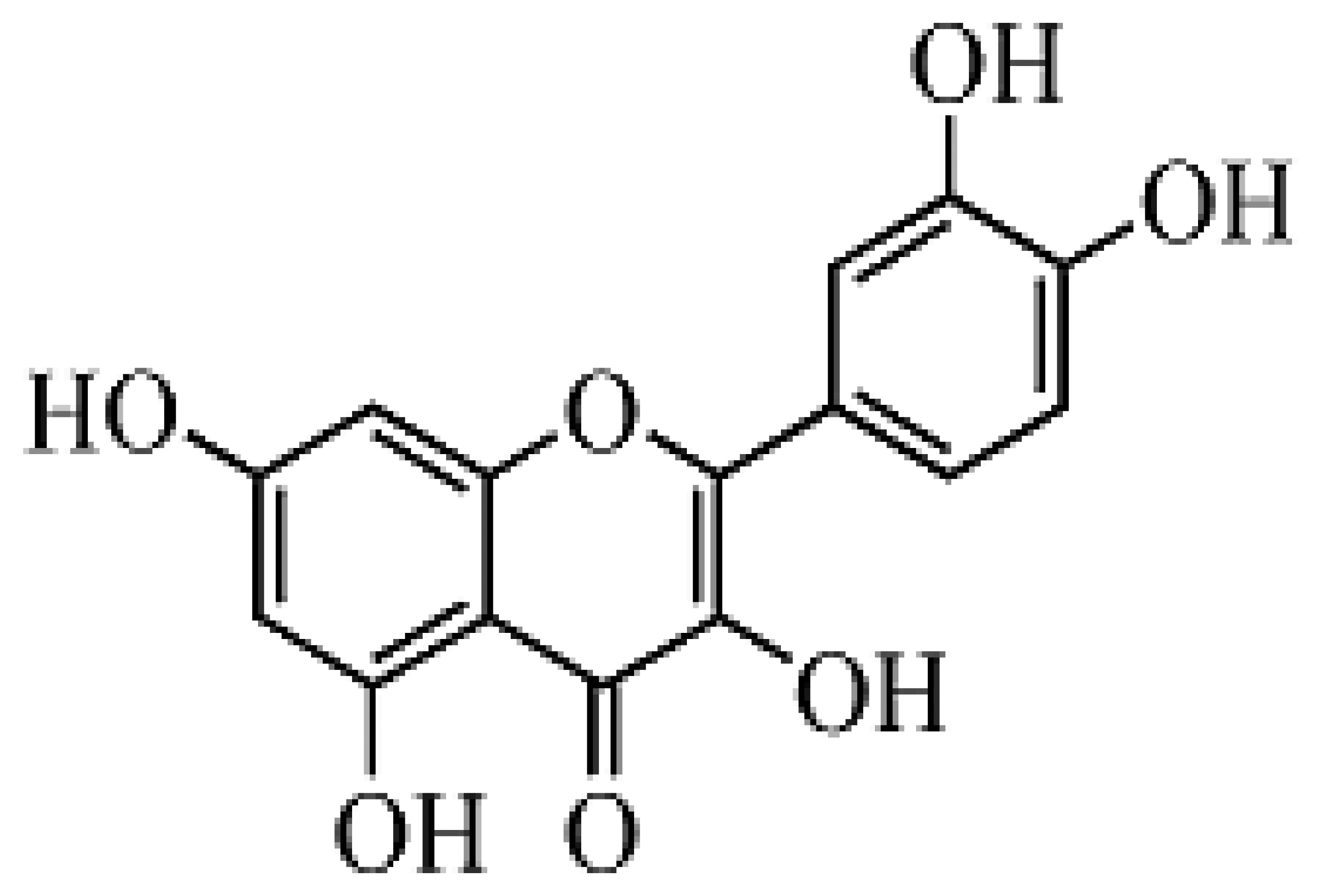
Flavonoids are natural products widely distributed in the plant kingdom and generally present in the common human diet. Evidence has shown that flavonoids are capable of modulating the activity of enzymes and affecting the behavior of many cell systems [1], and they are believed to be responsible for important health-enhancing effects [2]. Up to 2000, over 130 preparations containing certain kinds of flavonoids were registered as drugs worldwide [3]. Quercetin is the major representative of flavonol, one of the seven groups in the flavonoids family, a powerful antioxidant, and is regularly consumed by humans in edible fruits and vegetables at levels of up to 16 mg per day [4,5]. The molecular structure of quercetin is shown in Figure 1. Apart from flavonoids' therapeutic effects, quercetin is also excellent an scavenger of free radicals [6] which benefits humans immensely, therefore much attention has been paid to both the biological and physicochemical properties of quercetin over the past few decades. Hitherto, various analytical methods for the separation and determination of quercetin in flavonoids mixtures and real samples have been reported. High-performance liquid chromatography [7] and capillary electrophoresis [8–11] have been effectively used for the determination and quantification, coupled with various detection techniques [12], such as UV spectrophotometry, UV photodiode array detection, mass spectrometry, electrochemical detection and chemiluminescence. The above methods and their coupled techniques may provide high assay selectivity, but also leads to some disadvantages such as operational complexity, time and reagent consumption, high cost, etc. In fact, some direct methods for analysis of quercetin without pre-separation have also been described. They are mostly based on chemical derivitization combined with UV spectrophotometry [13], or a Kalman filter approach [14], but have a poor detection limit.
Because quercetin is electrochemically active, electrochemical methods with their advantages of higher sensitivity, low cost and less interferences from non-electroactive substances are preferable. The electrochemical oxidation of quercetin was studied by Nematollahi et al. [15] and Zare et al. [16] under different conditions. Conventional electrochemical methods with simple modification of the electrode surface can enable the determination of quercetin, despite the fact that strong absorption of quercetin on electrode surface leads to passivation of electrodes that hinders subsequent determination. Volikakis et al. [17] developed a way to detect quercetin on carbon paste electrodes through adsorptive stripping voltammetry. He et al. [18] utilized both the graphite-nujol paste electrode (GPE) and the carbon nanotubes-nujol paste electrode (CNTPE) for flavonoid determination. Recently, multiwall carbon nanotubes-modified electrodes have been found to be excellent electrodes for the determination of quercetin at trace levels due to their strong surface adsorption [19–21]. Electrodes modified with metal or metallic compounds are also used in quercetin detection [22–24].
The molecular imprinting technique (MIT) is becoming more and more commonly accepted as a useful method for recognition and isolation of key biological target molecules. When combined with template molecules, monomers may form multiple interactions, which are recorded through polymerization. After removal of template molecules, cavities come into being among the polymers which allow them to distinguish template molecules through their stereoconfiguration. Till now, it has been widely used in chromatographic [25], solid phase extraction [26], membrane separation [27], biological sensors [28] and electrochemical sensors [29,30], etc. Combined with MIT, the preparation of molecularly imprinted polymers (MIPs) in electrochemical sensors or biological sensors have considerable application prospects and important research significance [31,32]. Recently, there have been several studies on quercetin detection using MIT. Yu et al. [33] improved the selectivity of the quercetin MIP at low concentration. Pakade et al. [34] prepared quercetin MIPs in aqueous environment for selective recovery of quercetin from aqueous solutions at high temperature.
MIT has a great effect on improving the selectivity, but sensitivity is also an important factor for modified electrodes with excellent properties. It is proven that carbon-nanotubes and metallic nanoparticles contribute as well. Graphene now represents the new rising star in the field of material science and has attracted considerable attention in recent years. Structurally, graphene is a monolayer of tightly packed carbon atoms with a two-dimensional planar sheet of sp2-bonded carbon atoms arranged in a hexagonal configuration [35,36]. Since its experimental discovery in 2004, graphene has captured the interest of researchers because of its excellent properties, such as large surface-to-volume ratio, high conductivity and electron mobility at room temperature, robust mechanical properties, flexibility [37], good chemical stability, high transparency, and a broad electrochemical window [38]. Compared to tubular carbon nanotubes (CNTs), graphene oxide (GO) sheets could easily stick to glass carbon electrodes and fall off less easily. GO has a larger surface to volume ratio, a larger specific surface area, a greater atomic efficient utilization, and thus can improve electron transfer rates significantly. The unique structure and outstanding properties of graphene hold great promise for its applications, including photonic devices and photoluminescence [39], energy storage [40], photovoltaic devices [41,42], electrochemical supercapacitors [43] DNA-hybridization devices and drug delivery systems [44]. Because of their unique structural and electrical properties, graphene and graphene based materials can be fabricated as rapid and sensitive electrochemical sensors and nanodevices for small molecule determination, deoxyribonucleic acid (DNA) hybridization and protein–protein interactions [37,44]. In 2009, graphene was synthesized chemically by the Hummers and Offeman method and the graphene-modified electrode was first applied in selective determination of dopamine in a large excess of ascorbic acid [45]. From then on, graphene and grapheme-based materials have played important roles in the fabrication of chemical sensors as electrode modifiers. Combined with GO, several MIPs have been made into biochemical sensors [46,47]. Compared with other ways of synthesizing GO-MIP, electropolymerization methods have several advantages such as high fabrication rates, low material consumption and controllable film thickness, etc.
In this study, a method for determination of quercetin on a MIT/graphene modified glass carbon electrode was described. As far as we know, few paper about determination of quercetin using MIT on electrodes was reported [48,49]. In addition, this is the first time that GO was incorporated into a molecularly imprinted polymer for quercetin determination.
2. Experimental
2.1. Instruments and Materials
Electrochemical measurements were carried out on a model LK98B II microcomputer-based electrochemical analyzer (Lanlike Chemical High-tech Electronics Co. Ltd, Tianjin, China). A conventional three-electrode system was employed, including a working electrode, a platinum wire counter electrode and a saturated calomel electrode (SCE) reference electrode. Graphene oxide (purity >99%) was purchased from Vanguard Nanomaterial Reagent Co. Ltd. (Nanjing, China). Quercetin dehydrate, pyrrole, rutin trihydrate (Alfa Aesar, Tianjin, China), morin (Sigma-Aldrich, Shanghai, China) were of analytical grade and were used without further treatment. All aqueous solutions were prepared with reverse osmosis water (18.25 MΩ·cm).
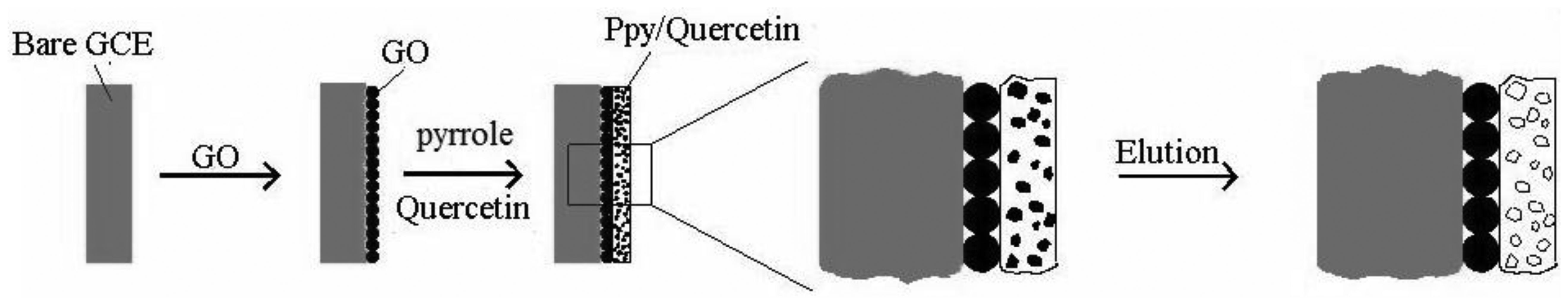
2.2. Fabrication of Molecularly Imprinted Polymer Incorporated Graphene Oxide-Modified Electrode with Polypyrrole (MIP/GO/GC)
Glass carbon electrodes (GC, Φ = 4 mm) were successively polished with 0.05 μm aluminum oxide slurries on chamois leather until a mirror-like surface was obtained. After rinsing with water in each polishing step, the electrodes were subject to ultra-sonication in water-ethanol mixture (1:1 v/v). Cyclic voltammetry(CV) has been employed to test the performance of the electrode under the following conditions: 1.0 × 10−3 mol/L K3Fe(CN)6, 0.1 mol/L phosphate buffer solution (pH = 7.0), with potential rate of 0.6 V to 0.2 V and a scan rate of 0.05 V/s. graphene oxide (GO, 2 mg) was dispersed into DMF (50 mL) by ultrasonic agitation for 10 min to give a homogeneous suspension. A certain portion of the GO-DMF suspension was transferred onto a clean GC surface and the suspension was dried under an infrared lamp. A GO/GC was thus fabricated. Electropolymerization in the three-electrode system in a solution which contains 0.05 mol/L pyrrole, 0.01 mol/L quercetin and 0.1 mol/L H2SO4 on GO/GC was carried out using chronocoulometry at 0.7 V for 100 s. Cyclic voltammetric experiments were performed on the modified electrode in 0.1 mol/L PBS (pH = 7.0) in a potential range from −0.2 to 1.3 V with a scan rate of 0.10 V/s for the over oxidation of polypyrrole film. Non-molecularly imprinted polymer electrode (NIP/GO/GC) was prepared as above, but without the template.
2.3. Electrochemical Determination of Quercetin with MIP/GO/GC
The working electrode was dipped into water-ethanol mixture (1:1 v/v) containing quercetin for a period of time for adsorption and then transferred to Britton-Robinson (B-R) buffer solution. Differential pulse voltammetry (DPV) experiments were performed in 0.1 mol/L B-R buffer solution (pH = 3.5). Experimental parameters were optimized according to the voltammetric response of quercetin in the solution.
3. Results and Discussion
3.1. Preparation of MIP/GO/GC
A MIP/GO/GC was fabricated as shown in Figure 2. Template molecules bound with functional monomer through hydrogen bonds. Under cyclic voltammetric scanning in a large range, hydrogen bonds were broken by potential change, therefore template molecules lose bonding force and were eluted. Electrochemical elution was preferable with its advantages of rapid response, good reproducibility, and furthermore resulted in the film's long-term stability.
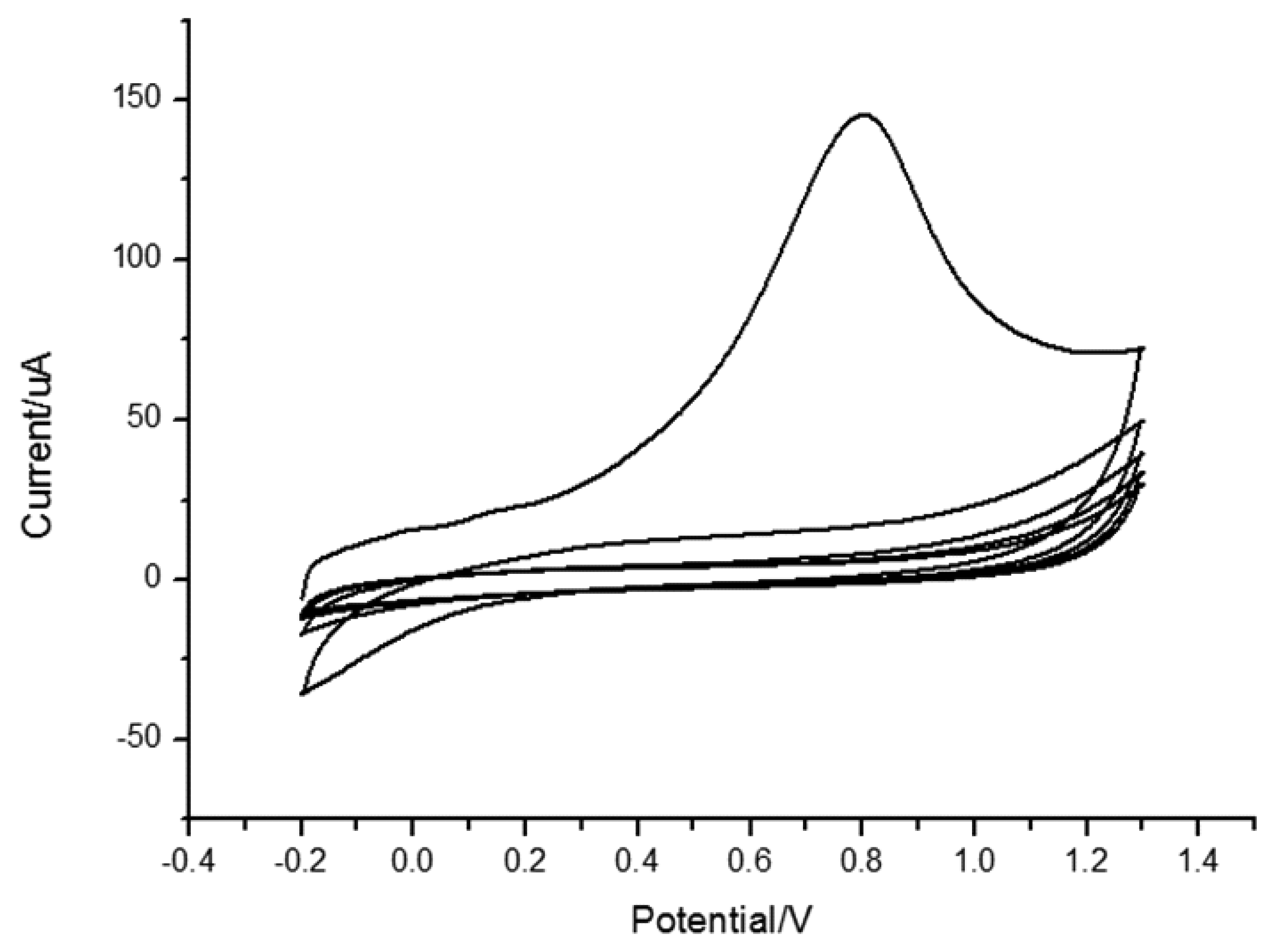
In 0.1 mol/L PBS (pH = 7.0), the electrochemical behavior of MIP/GO/GC was studied by using cyclic voltammetry in a scan range of −0.2 V and 1.3 V as shown in Figure 3. Compared with the first and the second cycle, the anodic peak at 0.8 V was disappeared at the second cycle, which indicated polypyrrole (PPy) was over oxidized.
Shiigi et al reported that the overoxidation of polypyrrole could improve the selectivity of molecularly imprinted film [50]. The polypyrrole skeleton will lose electroactivity after overoxidation under neutral or alkaline conditions while oxygen containing groups, such as carboxyl, are introduced into the skeleton. Under these conditions, template molecules are eluted from the polypyrrole film and thus a molecularly imprinted electrode is fabricated. Moreover, the anionic template is entrapped within the polymer during electropolymerization in the imprinting scheme based on overoxidized PPy. Subsequent overoxidation replaces the conventional washing step and creates nanocavities with template expulsion [31]. At the same time, a small peak belonging to quercetin at 0.2 V also vanished, which demonstrated that quercetin was eluted completely. The cyclic curves of the 4th and 5th cycle almost coincided with each other, which proved that the activation was complete.
3.2. Influence of Different GO contents
Different amounts of GO dispersed suspension were dropped onto the GC surface. After the electrode surface was dried, MIP/GO/GC and NIP/GO/GC were fabricated through electro-polymerization and overoxidation. Subsequently, MIP/GO/GC was eluted and activated, while NIP/GO/GC was directly activated. MIP/GO/GC and NIP/GO/GC containing different amounts of graphene were put into water-ethanol mixture (1:1 v/v) containing 5.0 × 10−6 mol/L quercetin for adsorption for 5 min. After rinsing with water, differential pulse voltammetry (DPV) method was used to determine quercetin in 0.1 mol/L Britton-Robinson (B-R) buffer solution (pH = 3.5). The electrochemical results are summarized in Table 1.
From the above table, we can see that the response current of MIP and NIP increased with increasing content of graphene because of the accelerated electron transfer rate of graphene. However, thick graphene layers would block electrons from reaching the electrode surface which leads to a current decrease at high graphene content. Based on the results listed in Table 1, 5.0 μL (0.2 μg) GO suspension was dropped on GC for the preparation of the electrodes.
3.3. Influence of Supporting Electrolyte and pH
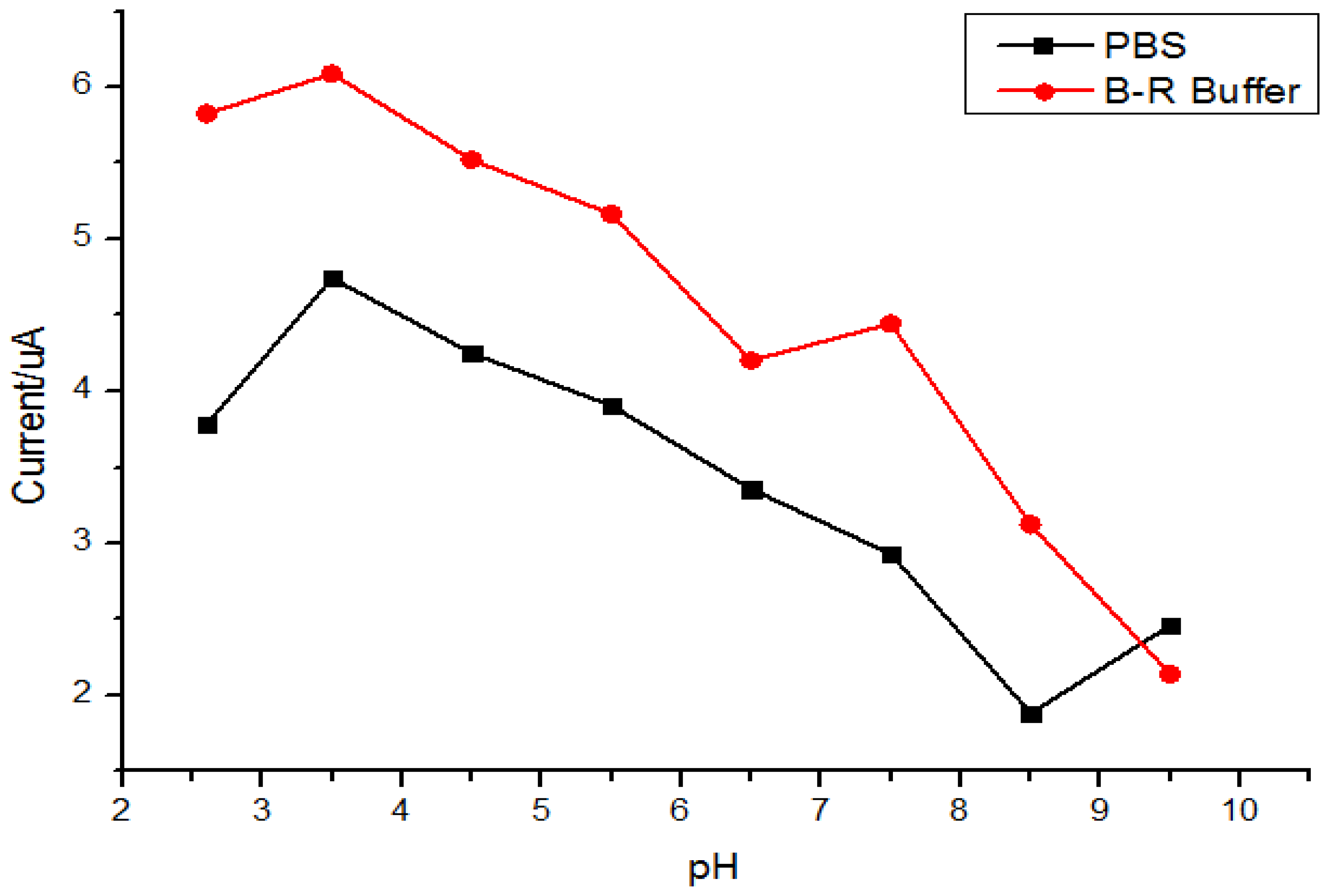
A series of buffer solutions with different pH values were prepared from 0.1 mol/L B-R or PBS solutions. MIP/GO/GC was put into water-ethanol mixture (1:1 v/v) containing 5.0 × 10−6 mol/L quercetin for adsorption for 5 min. After rinsing with water differential pulse voltammetry (DPV) experiments were carried out in the respective buffer solutions and the results are shown in Figure 4. The results indicated that a higher peak current and better peak shape could be obtained in a B-R buffer solution (pH = 3.5). This was attributed to the boric acid in the B-R buffer solution. Being electron-deficient atoms, boron atoms could attack the quercetin molecule effectively by electrostatic interactions with the hydroxyl group of quercetin. Moreover, poor response at high pH values could be due to deprotonation of polypyrrole [51].
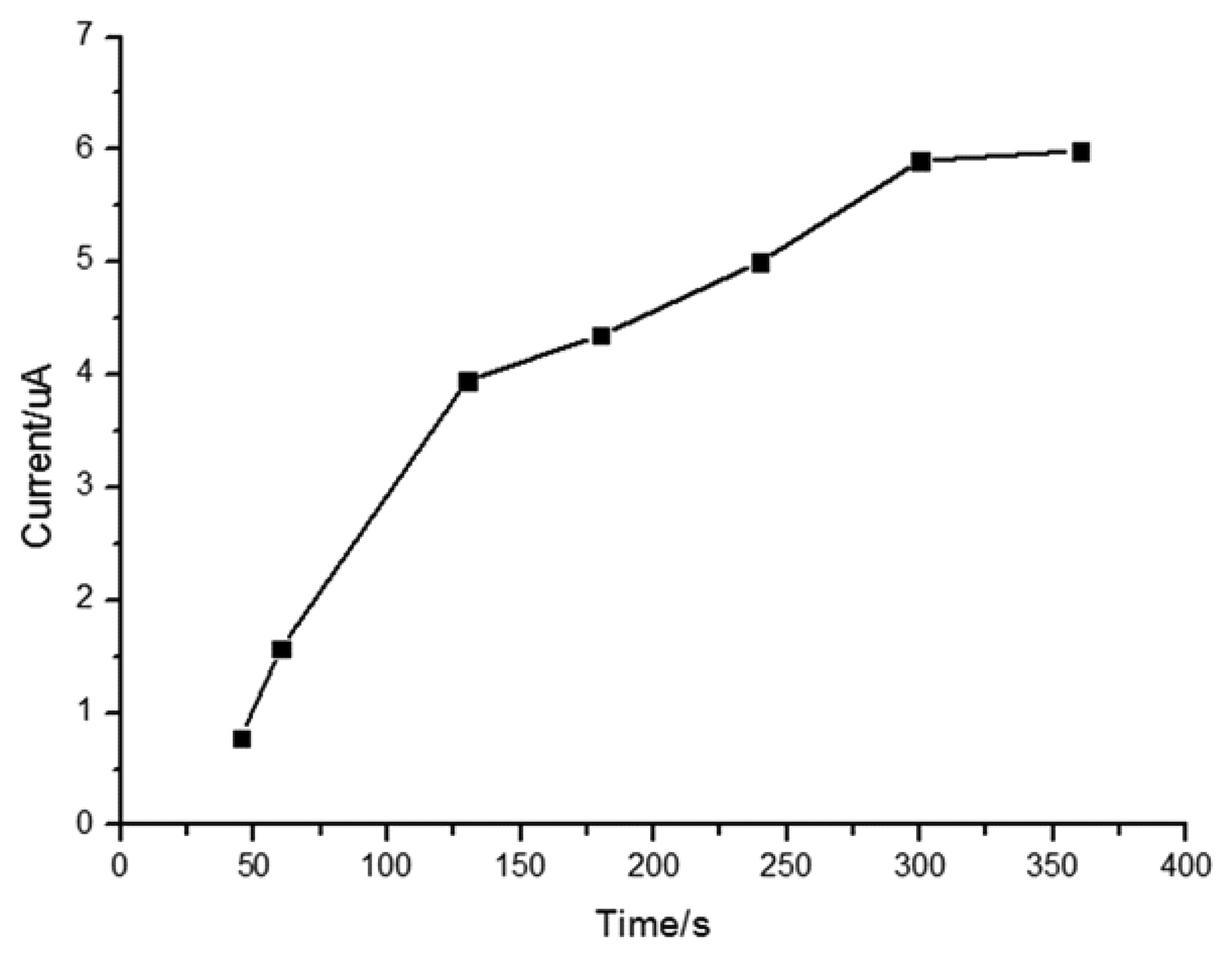
3.4. Influence of Adsorption Time
It has been reported that MIP films can reach absorption saturation in a short period of time [52,53]. Figure 5 shows that the peak current increases with adsorption time at the beginning of our experiments, but after five minutes, the peak current reaches a plateau value because of adsorption saturation, so the adsorption period of five minutes was selected for determination of quercetin.
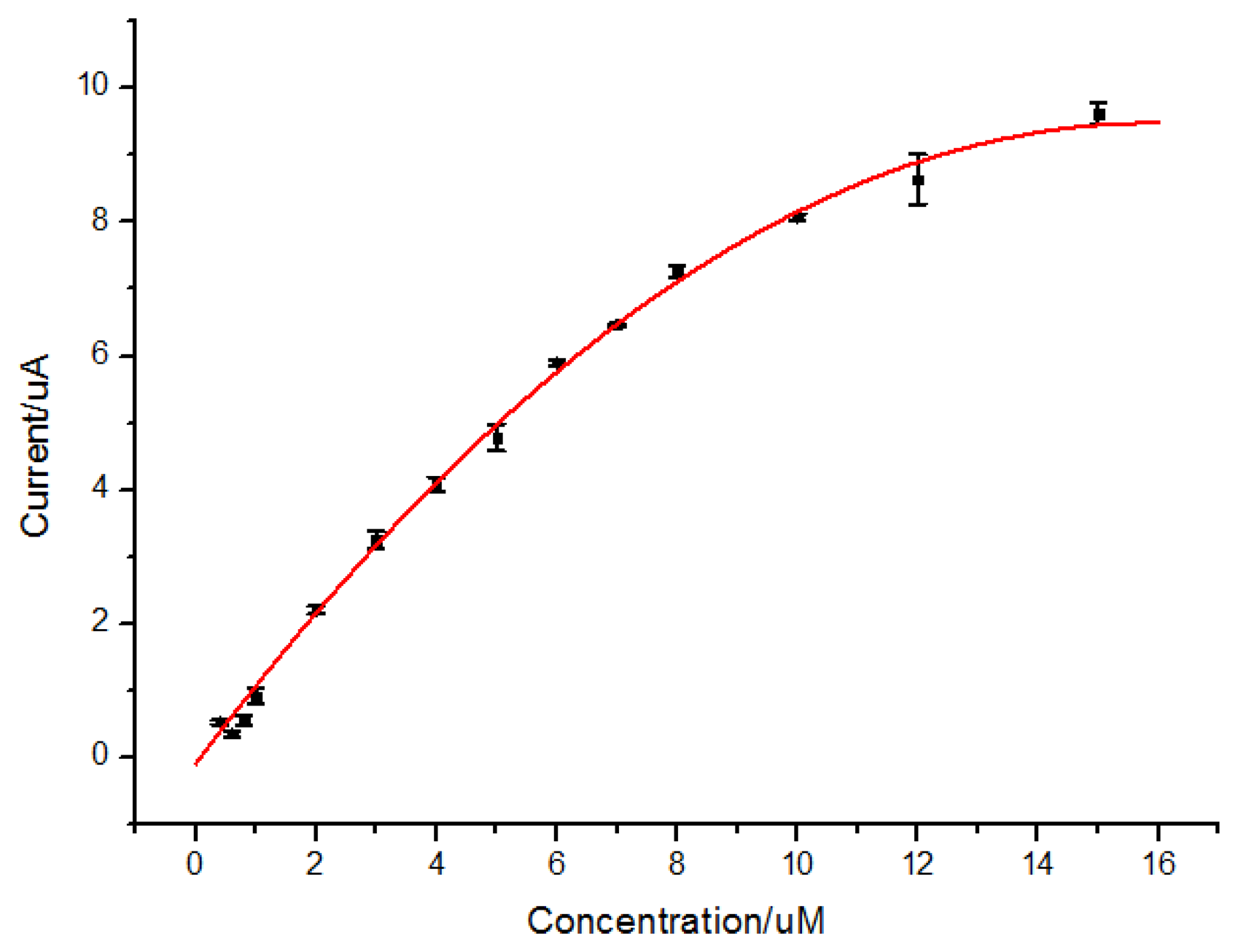
3.5. Calibration Curve
The DPV responses of quercetin in solutions in different concentrations were recorded as shown in Figure 6. The oxidation peak current of quercetin was dependent on the concentration of quercetin in the range from 6.0 × 10−7 to 1.5 × 10−5 mol/L (r2 = 0.997) by the equation of I(μA) = (−0.04 ± 0.003)C2(μmol2/L2) + (1.20 ± 0.033)C(μmol/L) + (−0.09 ± 0.075), with a detection limit of 4.8 × 10−8 mol/L (S/N = 3) as shown in Figure 7. The non-linear response of the electrodes may be attributed to both specific absorption and nonspecific absorption on the MIP/GO/GC electrodes.
3.6. Stability and Repeatability of MIP/GO/GC
Under the optimal conditions mentioned above, a 6.0 × 10−6 mol/L quercetin solution was measured successively for seven times with the same electrode. The relative standard deviation (RSD) of the peak current was 1.2%. Then the electrode was stored in water for 15 days. Afterwards, a 6.0 × 10−6 mol/L quercetin solution was investigated by the electrode under same experimental conditions for more than 300 scanning segments. The peak current decreased no more than 5%. In addition, five different MIP/GO/GC electrodes were prepared and tested in a 6.0 × 10−6 mol/L quercetin solution and the RSD of the peak current was 1.8%. The results indicated that MIP/GO/GC had good stability and repeatability.
3.7. Interferences
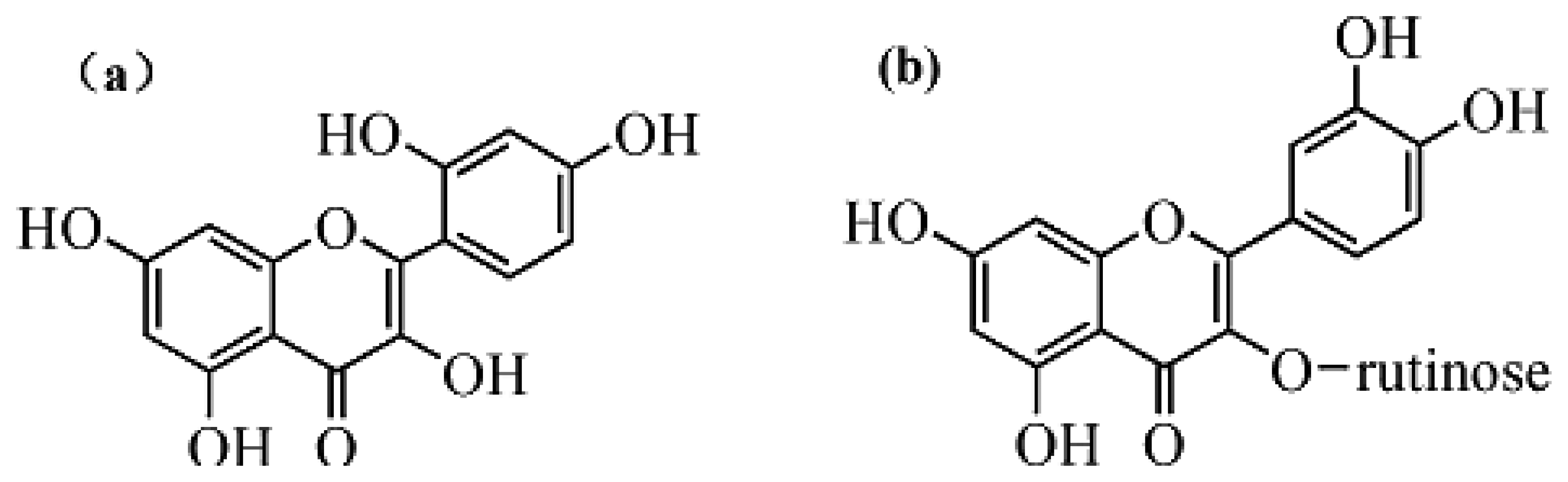
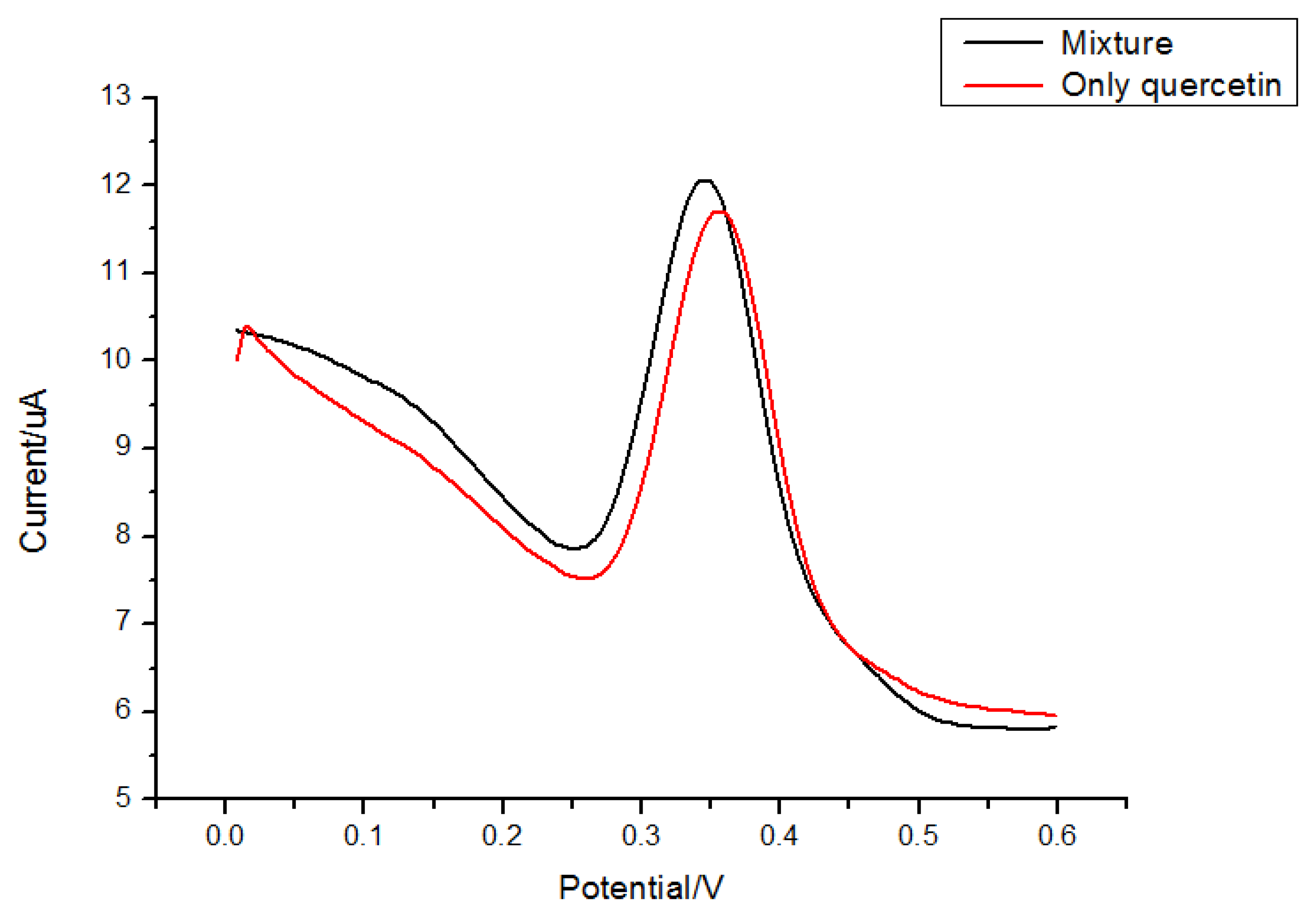
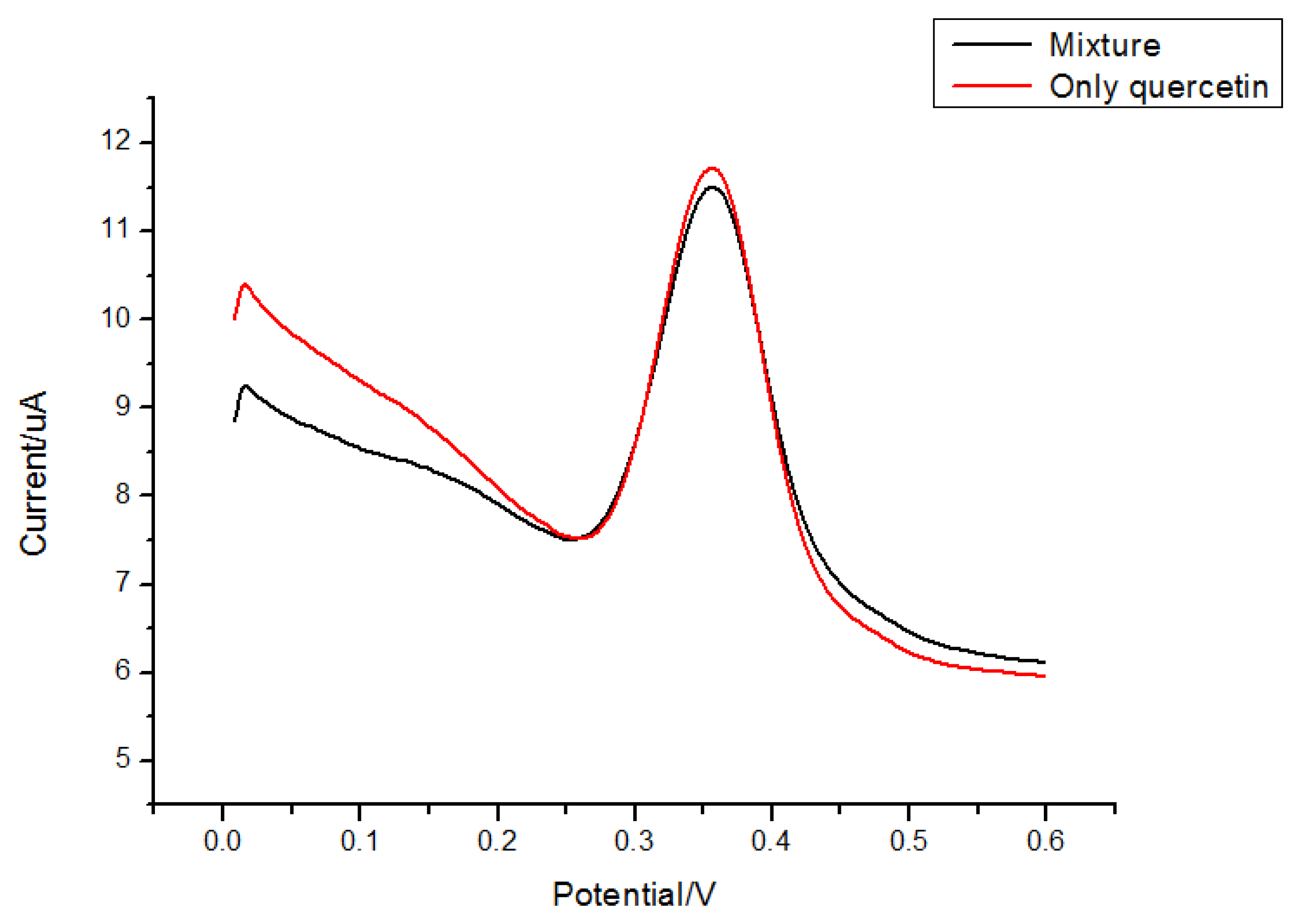
Rutin and morin (Figure 8) have similar structures as quercetin. Thus they were selected to test interferences in the proposed determination of quercetin. Considering the fact that the total contents of quercetin, rutin and morin are similar in most plants, quercetin solution was prepared by adding interferents with the same concentration, so a mixed solution of 5.0 × 10−6 mol/L morin and 5.0 × 10−6 mol/L quercetin was tested for the interference study. Results (Figures 9 and 10) showed that 5.0 × 10−6 mol/L morin or rutin would not affect the determination of quercetin at the same concentration. In this sense, the electrode proposed in this paper had a high selectivity.
3.8. Analysis of Quercetin in Complex Matrices
Fruit juices usually contain quercetin in the range from 2.96 × 10−6 to 3.84 × 10−5 mol/L. Newly expressed apple juice (10.0 mL) was used as a real sample and was diluted five times with water-ethanol mixture (1:1 v/v) before the determination. The quercetin concentrations in our samples were calculated using the standard addition method under optimized conditions. The results shown in Table 2, indicate that MIP/GO/GC could be used in the determination of quercetin in complex matrices with satisfactory results.
4. Conclusions
Graphene oxide has a large surface-to-volume ratio and high electron transmission rate which improves significantly the sensitivity of electrochemically modified electrodes based on graphene. MIT can increase the selectivity of modified electrode considerably. MIP/GO/GC could be obtained simply by electropolymerization of pyrrole in the presence of the template (quercetin) on graphene oxide modified electrode. MIP/GO/GC has a polynomial response in the range from 6.0 × 10−7 to 1.5 × 10−5 mol/L (r2 = 0.997) with a detection limit of 4.8 × 10−8 mol/L (S/N = 3). The selectivity of MIP/GO/GC was tested by using rutin and morin which have similar structures. The results showed that MIP/GO/GC had a good imprinting effect for the recognition of quercetin. The application of MIP/GO/GC was also tested by determining quercetin in newly expressed apple juice.
Acknowledgments
Financial support from National Natural Science Foundation of China (21075067) and the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201204) were greatly appreciated.
References
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar]
- Kao, T.H.; Huang, S.C.; Inbaraj, B.S.; Chen, B.H. Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2008, 626, 200–211. [Google Scholar]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Maenpaa, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar]
- Manach, C.; Morand, C.; Crespy, V.; Demigne, C.; Texier, O.; Regerat, F.; Remesy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336. [Google Scholar]
- Prior, R.L. Absorption and Metabolism of Anthocyanins: Potential Health Effects. Proceedings of the 4th International Phytochemical Conference, Pomona, CA, USA, 21– 22 October 2002.
- Van Acker, S.A.; Tromp, M.N.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Flavonoids as scavengers of nitric oxide radical. Biochem. Biophys. Res. Commun. 1995, 214, 755–759. [Google Scholar]
- Berger, L.M.; Wein, S.; Blank, R.; Metges, C.C.; Wolffram, S. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J. Dairy Sci. 2012, 95, 5047–5055. [Google Scholar]
- Chen, G.; Zhang, H.W.; Ye, J.N. Determination of baicalein, baicalin and quercetin in scutellariae radix and its preparations by capillary electrophoresis with electrochemical detection. Talanta 2000, 53, 471–479. [Google Scholar]
- Chen, G.; Zhang, H.W.; Ye, J.N. Determination of rutin and quercetin in plants by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta 2000, 423, 69–76. [Google Scholar]
- Cao, Y.H.; Zhang, X.; Fang, Y.H.; Ye, J.N. Determination of active ingredients of Apocynum Venetum by capillary electrophoresis with electrochemical detection. Mikrochim. Acta 2001, 137, 57–62. [Google Scholar]
- Sun, Y.; Guo, T.; Sui, Y.; Li, F.M. Quantitative determination of rutin, quercetin, and adenosine in Flos Carthami by capillary electrophoresis. J. Sep. Sci. 2003, 26, 1203–1206. [Google Scholar]
- Dmitrienko, S.G.; Kudrinskaya, V.A.; Apyari, V.V. Methods of extraction, preconcentration, and determination of quercetin. J. Anal. Chem. 2012, 67, 299–311. [Google Scholar]
- Baranowska, I.; Rarog, D. Application of derivative spectrophotometry to determination of flavonoid mixtures. Talanta 2001, 55, 209–212. [Google Scholar]
- Hassan, H.N.A.; Barsoum, B.N.; Habib, I.H.I. Simultaneous spectrophotometric determination of rutin, quercetin and ascorbic acid in drugs using a kalman filter approach. J. Pharm. Biomed. 1999, 20, 315–320. [Google Scholar]
- Nematollahi, D.; Malakzadeh, M. Electrochemical oxidation of quercetin in the presence of benzenesulfinic acids. J. Electroanal. Chem. 2003, 547, 191–195. [Google Scholar]
- Zare, H.R.; Namazian, M.; Nasirizadeh, N. Electrochemical behavior of quercetin: Experimental and theoretical studies. J. Electroanal. Chem. 2005, 584, 77–83. [Google Scholar]
- Volikakis, G.J.; Efstathiou, C.E. Determination of rutin and other flavonoids by flow-injection/adsorptive stripping voltammetry using nujol-graphite and diphenylether-graphite paste electrodes. Talanta 2000, 51, 775–785. [Google Scholar]
- He, J.B.; Lin, X.Q.; Pan, J. Multi-wall carbon nanotube paste electrode for adsorptive stripping determination of quercetin: A comparison with graphite paste electrode via voltammetry and chronopotentiometry. Electroanal 2005, 17, 1681–1686. [Google Scholar]
- Xiao, P.; Zhao, F.Q.; Zeng, B.Z. Voltammetric determination of quercetin at a multi-walled carbon nanotubes paste electrode. Microchem. J 2007, 85, 244–249. [Google Scholar]
- Xu, G.R.; Kim, S. Selective determination of quercetin using carbon nanotube-modified electrodes. Electroanal 2006, 18, 1786–1792. [Google Scholar]
- Gutierrez, F.; Ortega, G.; Cabrera, J.L.; Rubianes, M.D.; Rivas, G.A. Quantification of quercetin using glassy carbon electrodes modified with multiwalled carbon nanotubes dispersed in polyethylenimine and polyacrylic acid. Electroanal 2010, 22, 2650–2657. [Google Scholar]
- Franzoi, A.C.; Vieira, I.C.; Scheeren, C.W.; Dupont, J. Development of quercetin biosensor through immobilizing laccase in a modified beta-cyclodextrin matrix containing Ag nanoparticles in ionic liquid. Electroanal 2010, 22, 1376–1385. [Google Scholar]
- Oliveira, A.C.; Mascaro, L.H. Evaluation of carbon nanotube paste electrode modified with copper microparticles and its application to determination of quercetin. Int. J. Electrochem. Sci. 2011, 6, 804–818. [Google Scholar]
- Wang, M.Y.; Zhang, D.E.; Tong, Z.W.; Xu, X.Y.; Yang, X.J. Voltammetric behavior and the determination of quercetin at a flowerlike Co3O4 nanoparticles modified glassy carbon electrode. J. Appl. Electrochem 2011, 41, 189–196. [Google Scholar]
- Khorrami, A.R.; Rashidpur, A. Development of a fiber coating based on molecular sol-gel imprinting technology for selective solid-phase micro extraction of caffeine from human serum and determination by gas chromatography/mass spectrometry. Anal. Chim. Acta 2012, 727, 20–25. [Google Scholar]
- Ameli, A.; Alizadeh, N. Nanostructured conducting molecularly imprinted polymer for selective extraction of salicylate from urine and serum samples by electrochemically controlled solid-phase micro-extraction. Anal. Chim. Acta 2011, 707, 62–68. [Google Scholar]
- Ulbricht, M. Membrane separations using molecularly imprinted polymers. J. Chromatogr. B. 2004, 804, 113–125. [Google Scholar]
- Yang, Z.P.; Zhang, C.J. Molecularly imprinted hydroxyapatite thin film for bilirubin recognition. Biosens. Bioelectron. 2011, 29, 167–171. [Google Scholar]
- Malitesta, C.; Losito, I.; Zambonin, P.G. Molecularly imprinted electrosynthesized polymers: New materials for biomimetic sensors. Anal. Chem. 1999, 71, 1366–1370. [Google Scholar]
- Liang, H.J.; Ling, T.R.; Rick, J.F.; Chou, T.C. Molecularly imprinted electrochemical sensor able to enantroselectivly recognize D and L-tyrosine. Anal. Chim. Acta 2005, 542, 83–89. [Google Scholar]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. Mip sensors—the electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar]
- Suryanarayanan, V.; Wu, C.T.; Ho, K.C. Molecularly imprinted electrochemical sensors. Electroanal 2010, 22, 1795–1811. [Google Scholar]
- Yu, H.; Chen, Z.B.; Fu, Y.; Kang, L.; Wang, M.; Du, X.Y. Synthesis and optimization of molecularly imprinted polymers for quercetin. Polym. Int. 2012, 61, 1002–1009. [Google Scholar]
- Pakade, V.; Lindahl, S.; Chimuka, L.; Turner, C. Molecularly imprinted polymers targeting quercetin in high-temperature aqueous solutions. J. Chromatogr. A 2012, 1230, 15–23. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Obergfell, D.; Roth, S.; Girit, C.; Zettl, A. On the roughness of single- and bi-layer graphene membranes. Solid State Commun. 2007, 143, 101–109. [Google Scholar]
- Gan, T.; Hu, S.S. Electrochemical sensors based on graphene materials. Microchim. Acta 2011, 175, 1–19. [Google Scholar]
- Li, Z.J.; Yang, B.C.; Zhang, S.R.; Zhao, C.M. Graphene oxide with improved electrical conductivity for supercapacitor electrodes. Appl. Surf. Sci. 2012, 258, 3726–3731. [Google Scholar]
- Blake, P.; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W.; et al. Graphene-based liquid crystal device. Nano Lett. 2008, 8, 1704–1708. [Google Scholar]
- Zhang, Y.B.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry's phase in graphene. Nature 2005, 438, 201–204. [Google Scholar]
- Guo, C.X.; Yang, H.B.; Sheng, Z.M.; Lu, Z.S.; Song, Q.L.; Li, C.M. Layered graphene/quantum dots for photovoltaic devices. Angew. Chem. Int. Edit. 2010, 49, 3014–3017. [Google Scholar]
- Lu, Z.S.; Guo, C.X.; Yang, H.B.; Qiao, Y.; Guo, J.; Li, C.M. One-step aqueous synthesis of graphene-CdTe quantum dot-composed nanosheet and its enhanced photoresponses. J. Colloid. Interf. Sci. 2011, 353, 588–592. [Google Scholar]
- Liu, W.W.; Yan, X.B.; Lang, J.W.; Chen, J.T.; Xue, Q.J. Influences of the thickness of self-assembled graphene multilayer films on the supercapacitive performance. Electrochim. Acta 2012, 60, 41–49. [Google Scholar]
- Giovanni, M.; Bonanni, A.; Pumera, M. Detection of DNA hybridization on chemically modified graphene platforms. Analyst 2012, 137, 580–583. [Google Scholar]
- Wang, Y.; Li, Y.M.; Tang, L.H.; Lu, J.; Li, J.H. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar]
- Mao, Y.; Bao, Y.; Gan, S.Y.; Li, F.H.; Niu, L. Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens. Bioelectron. 2011, 28, 291–297. [Google Scholar]
- Liu, Y.; Zhu, L.H.; Zhang, Y.Y.; Tang, H.Q. Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer. Sens. Actuators B Chem. 2012, 171, 1151–1158. [Google Scholar]
- Zhu, L.Y.; Wang, Z.H.; Chen, X.Y.; Zhang, F.F.; Xia, Y.Z.; Li, Y.H. Molecularly imprinted polymer incorporated carbon nanotube modified electrode for selective determination of quercetin. J. Instrum. Anal. 2011, 30, 18–23. [Google Scholar]
- Gam-Derouich, S.; Nguyen, M.N.; Madani, A.; Maouche, N.; Lang, P.; Perruchot, C.; Chehimi, M.M. Aryl diazonium salt surface chemistry and ATRP for the preparation of molecularly imprinted polymer grafts on gold substrates. Surf. Int. Anal. 2010, 42, 1050–1056. [Google Scholar]
- Shiigi, H.; Yakabe, H.; Kishimoto, M.; Kijima, D.; Zhang, Y.A.; Sree, U.; Deore, B.A.; Nagaoka, T. Molecularly imprinted overoxidized polypyrrole colloids: Promising materials for molecular recognition. Microchim. Acta 2003, 143, 155–162. [Google Scholar]
- Saoudi, B.; Jammul, N.; Abel, M.; Chehimi, M.; Dodin, G. DNA adsorption onto conducting polypyrrole. Synth. Metals 1997, 87, 97–103. [Google Scholar]
- Xu, X.L.; Zhou, G.L.; Li, H.X.; Liu, Q.; Zhang, S.; Kong, J.L. A novel molecularly imprinted sensor for selectively probing imipramine created on ito electrodes modified by au nanoparticles. Talanta 2009, 78, 26–32. [Google Scholar]
- Maouche, N.; Guergouri, M.; Gam-Derouich, S.; Jouini, M.; Nessark, B.; Chehimi, M.M. Molecularly imprinted polypyrrole films: Some key parameters for electrochemical picomolar detection of dopamine. J. Electroanal. Chem. 2012, 685, 21–27. [Google Scholar]

| Volume of GO Dispersed Suspension Dropped on the Electrode Surface(μL) | NIP/GO/GC (μA) | MIP (μA) | Imprinting Factor |
|---|---|---|---|
| 0.0 | 0.00 | 0.55 | |
| 2.0 | 0.79 | 0.89 | 1.13 |
| 3.0 | 0.72 | 1.52 | 2.11 |
| 4.0 | 0.84 | 2.47 | 2.94 |
| 5.0 | 0.79 | 4.48 | 5.67 |
| 5.5 | 0.89 | 2.25 | 2.53 |
| 6.0 | 0.86 | 1.97 | 2.29 |
| Addition (μmol/L) | Measured (μmol/L) | Recovery (%) | RSD (n=3) (%) |
|---|---|---|---|
| 0.000 | 0.759 | - | - |
| 1.994 | 2.791 | 101.4 | 1.40 |
| 2.989 | 3.689 | 98.4 | 1.60 |
| 3.485 | 4.239 | 99.9 | 0.52 |
| 3.981 | 4.619 | 97.4 | 1.29 |
| 4.476 | 5.260 | 100.5 | 2.89 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/
Share and Cite
Sun, S.; Zhang, M.; Li, Y.; He, X. A Molecularly Imprinted Polymer with Incorporated Graphene Oxide for Electrochemical Determination of Quercetin. Sensors 2013, 13, 5493-5506. https://doi.org/10.3390/s130505493
Sun S, Zhang M, Li Y, He X. A Molecularly Imprinted Polymer with Incorporated Graphene Oxide for Electrochemical Determination of Quercetin. Sensors. 2013; 13(5):5493-5506. https://doi.org/10.3390/s130505493
Chicago/Turabian StyleSun, Si, Mengqi Zhang, Yijun Li, and Xiwen He. 2013. "A Molecularly Imprinted Polymer with Incorporated Graphene Oxide for Electrochemical Determination of Quercetin" Sensors 13, no. 5: 5493-5506. https://doi.org/10.3390/s130505493




